
HL Paper 2
This question is about the reactions of halogenoalkanes.
Compare and contrast the mechanisms by which 1-chlorobutane, CH3CH2CH2CH2Cl, and 2-chloro-2-methylpropane, (CH3)3CCl, react with aqueous sodium hydroxide, giving two similarities and one difference.
Outline why the rate of reaction of the similar bromo-compounds is faster.
State the organic product of the reaction between 1-chlorobutane, CH3CH2CH2CH2Cl, and aqueous sodium hydroxide.
Suggest how this product could be synthesized in one step from butanoic acid.
Deduce the name of the class of compound formed when the product of (c)(i) reacts with butanoic acid.
Markscheme
Any two similarities:
heterolytic bond breaking
OR
chloride ions leave
nucleophilic/OH– substitution
both first order with regard to [halogenoalkane]
One difference:
CH3CH2CH2CH2Cl is second order/bimolecular/SN2 AND (CH3)3CCl is first order/unimolecular/SN1
OR
CH3CH2CH2CH2Cl rate depends on [OH–] AND (CH3)3CCl does not
OR
CH3CH2CH2CH2Cl is one step AND (CH3)3CCl is two steps
OR
(CH3)3CCl involves an intermediate AND CH3CH2CH2CH2Cl does not
OR
CH3CH2CH2CH2Cl has inversion of configuration AND (CH3)3CCl has c. 50 : 50 retention and inversion
Do not accept “produces alcohol” or “produces NaCl”.
Accept “substitution in 1-chlorobutane and «some» elimination in 2-chloro-2-methylpropane”.
[3 marks]
C–Br bond weaker than C–Cl bond
Accept “Br– is a better leaving group”.
Do not accept "bromine is more reactive".
Do not accept “C–Br bond is longer than C–Cl” alone.
[1 mark]
butan-1-ol/CH3CH2CH2CH2OH
Do not accept “butanol” for “butan-1-ol”.
Accept “1-butanol”.
Do not penalize for name if correct formula is drawn.
[1 mark]
«reduction with» lithium aluminium hydride/LiAlH4
Do not accept “sodium borohydride/NaBH4”.
[1 mark]
ester
[1 mark]
Examiners report
Geometrical isomerism and optical isomerism are two sub-groups of stereoisomerism in organic chemistry.
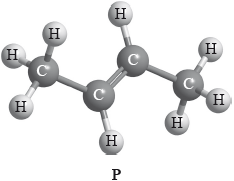
Compound P has the following three-dimensional structure. P also has geometrical isomers.
Menthol can be used in cough medicines. The compound contains C, H and O only.
Describe what is meant by the term stereoisomers.
Geometrical isomers have different physical properties and many drugs, such as doxepin (which has antidepressant properties), have geometrical isomers.
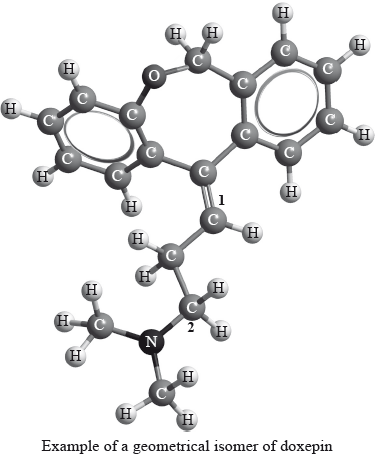
For each of the carbon atoms labelled 1 and 2 in doxepin, deduce the type of hybridization involved (sp, sp2 or sp3).
1:
2:
Clomifene, a fertility drug, whose three-dimensional structure is represented below, also has geometrical isomers.
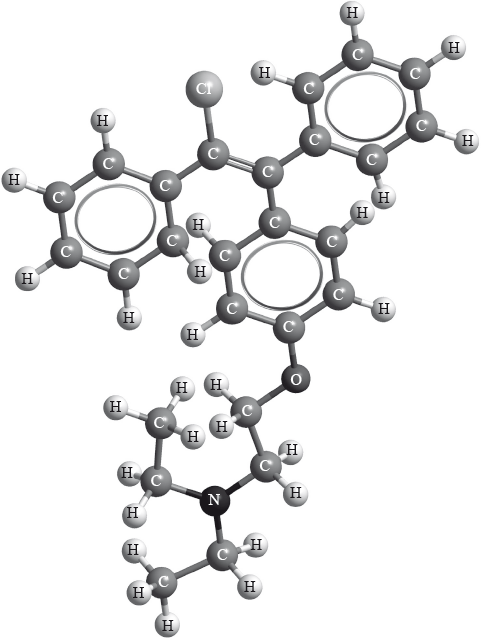
Identify the name of one functional group present in clomifene.
Draw any two other isomers of P.
Apply IUPAC rules to state the names of all the straight-chain isomers of compounds of molecular formula C4H8 (including P).
State the structural formula of the organic products, Q, R, S and T, formed in the following reactions.

Suggest one suitable mechanism for the reaction of Q with aqueous sodium hydroxide to form T, using curly arrows to represent the movement of electron pairs.
State the structural formula of the organic product formed, U, when R is heated under reflux with acidified potassium dichromate(VI).
Apply IUPAC rules to state the name of this product, U.
When a \(6.234 \times {10^{ - 2}}{\text{ g}}\) of the compound was combusted, \(1.755 \times {10^{ - 1}}{\text{ g}}\) of carbon dioxide and \(7.187 \times {10^{ - 2}}{\text{ g}}\) of water were produced. Determine the molecular formula of the compound showing your working, given that its molar mass is \(M = 156.30{\text{ g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
Menthol occurs naturally and has several isomers. State the structural feature of menthol which is responsible for it having enantiomers.
State the instrument used to distinguish between each of the two enantiomers, and how they could be distinguished using this instrument.
Compare the physical and chemical properties of enantiomers.
Physical properties:
Chemical properties:
Markscheme
compounds with same structural formula but different arrangements of atoms in space;
Award [1] if correct description of geometric and optical isomers given.
1: sp2 and 2: sp3;
amine;
benzene ring;
Allow phenyl (group).
Do not allow just benzene.
alkene / chloroalkene;
chloro;
ether / phenyl ether;
Ethers not required as per guide but allow if given.
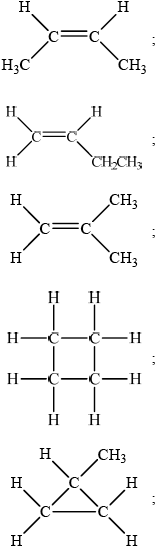
trans-but-2-ene and cis-but-2-ene;
Allow trans 2-butene and cis 2-butene.
Do not accept just 2-butene or 2-butene.
but-1-ene;
Allow 1-butene.
Q: \({\text{C}}{{\text{H}}_3}{\text{CHBrC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}\);
R: \({\text{C}}{{\text{H}}_3}{\text{CH(OH)C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}\);
S: \({\text{C}}{{\text{H}}_3}{\text{CHBrCHBrC}}{{\text{H}}_3}\);
T: \({\text{C}}{{\text{H}}_3}{\text{CH(OH)C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}\);
Condensed or full structural formulas may be given.
Since secondary bromoalkane could be either SN1 and SN2 so allow SN1 or SN2 for M1 –M4.
SN1:
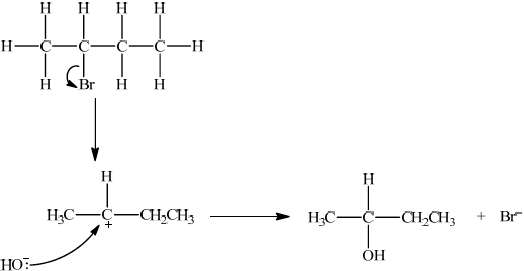
curly arrow showing Br leaving;
Do not allow arrow originating from C to C–Br bond.
representation of secondary carbocation;
curly arrow going from lone pair/negative charge on O in \({\text{H}}{{\text{O}}^ - }\) to \({{\text{C}}^ + }\);
Do not allow arrow originating on H in OH–.
formation of \({\text{C}}{{\text{H}}_3}{\text{CH(OH)C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}\) and \({\text{B}}{{\text{r}}^ - }\);
Allow formation of NaBr instead of Br–.
OR
SN2:
curly arrow going from lone pair/negative charge on O in \({\text{H}}{{\text{O}}^ - }\) to C;
Do not allow curly arrow originating on H in OH–.
curly arrow showing Br leaving;
Accept curly arrow either going from bond between C and Br to Br in 2-bromobutane or in the transition state.
Do not allow arrow originating from C to C–Br bond.
representation of transition state showing negative charge, square brackets and partial bonds;
Do not penalize if HO and Br are not at 180° to each other.
Do not award M3 if OH—C bond is represented.
formation of \({\text{C}}{{\text{H}}_3}{\text{CH(OH)C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}\) and \({\text{B}}{{\text{r}}^ - }\);
Allow formation of NaBr instead of Br–.
\({{\text{H}}_3}{\text{CCOC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}\);
Condensed or full structural formula may be given.
butan-2-one;
Allow 2-butanone or butanone.
Accept butan-2-one if (v) is incorrect but also apply ECF.
\({m_{\text{C}}}:(1.755 \times {10^{ - 1}} \times 12.01)/(44.01) = 4.790 \times {10^{ - 2}}{\text{ g}}\) and
\({m_{\text{H}}}:(7.187 \times {10^{ - 2}} \times 2 \times 1.01)/(18.02) = 8.056 \times {10^{ - 3}}{\text{ g}}\);
\({m_{\text{O}}}:(6.234 \times {10^{ - 2}} - 8.056 \times {10^{ - 3}} - 4.790 \times {10^{ - 2}}) = 6.384 \times {10^{ - 3}}{\text{ g}}\);
\(({n_{\text{C}}} = 3.988 \times {10^{ - 3}}\) and \({n_{\text{H}}} = 2 \times 3.988 \times {10^{ - 3}}\) and \({n_{\text{O}}} = 3.988 \times {10^{ - 3}}\) hence empirical formula \( = {\text{) }}{{\text{C}}_{10}}{{\text{H}}_{20}}{\text{O}}\);
\(\left( {M{\text{(}}{{\text{C}}_{10}}{{\text{H}}_{20}}{\text{O)}} = 156.30{\text{ (g}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{), therefore molecular formula}} = } \right){\text{ }}{{\text{C}}_{10}}{{\text{H}}_{20}}{\text{O}}\);
OR
\({n_{{\text{C}}{{\text{O}}_2}}} = \left( {\frac{{1.755 \times {{10}^{ - 1}}}}{{44.01}}} \right) = 3.988 \times {10^{ - 3}}\) and \({n_{{{\text{H}}_2}{\text{O}}}} = \left( {\frac{{7.187 \times {{10}^{ - 1}}}}{{18.02}}} \right) = 3.988 \times {10^{ - 3}}\);
\({m_{\text{O}}}:(6.234 \times {10^{ - 2}} - 8.056 \times {10^{ - 3}} - 4.790 \times {10^{ - 2}}) = 6.384 \times {10^{ - 3}}{\text{ g}}\);
\(({n_{\text{C}}} = 3.988 \times {10^{ - 3}}\) and \({n_{\text{H}}} = 2 \times 3.988 \times {10^{ - 3}}\) and \({n_{\text{O}}} = 3.988 \times {10^{ - 3}}\) hence empirical formula \( = {\text{) }}{{\text{C}}_{{\text{10}}}}{{\text{H}}_{20}}{\text{O}}\);
\(\left( {M{\text{(}}{{\text{C}}_{10}}{{\text{H}}_{20}}{\text{O)}} = 156.30{\text{ (g}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{), therefore molecular formula }} = } \right){\text{ }}{{\text{C}}_{10}}{{\text{H}}_{20}}{\text{O}}\);
Allow alternative working to be used.
Award [3 max] for C10H20O if no working shown.
chiral (carbon/centre/atom) / (tetrahedral) carbon surrounded by four
different groups;
Accept chiral compound or chiral molecule.
polarimeter and (enantiomers) rotate plane of polarized light in (equal and) opposite directions;
Physical properties:
identical except for rotation of plane polarized light;
Accept “identical” as different optical properties assessed in (iii).
Do not accept similar.
Chemical properties:
identical unless they interact with other optically active/chiral compounds/reagents/solvents / identical with achiral compounds/reagents/solvents / OWTTE;
Allow different physiological effects/taste.
Examiners report
A reasonably popular question and often well done. In (a), some weaker candidates did not understand the idea of a stereoisomer.
(b) and (c) were well done.
(b) and (c) were well done.
In (d), most scored full marks though some gave cis.
In (d), most scored full marks though some gave cis. In (ii), many did not gain marks for but-2-ene.
In (d), most scored full marks though some gave cis.
In (d), most scored full marks though some gave cis.
In (d), most scored full marks though some gave cis.
In (d), most scored full marks though some gave cis.
(e) (i) also was very well answered compared to some recent sessions.
Perhaps too much was expected in (iii) for one mark and students either omitted polarimeter or did not refer to plane polarised light.
In (iv), few scored both marks.
But-2-ene is a straight-chain alkene with formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}\). The molecule contains both \(\sigma \) and \(\pi \) bonds.

The polymerization of the alkenes is one of the most significant reactions of the twentieth century.
(i) Explain the formation of the \(\pi \) bond.
(ii) For each of the carbon atoms, C(1) and C(2), identify the type of hybridization shown.
C(1):
C(2):
But-2-ene shows geometrical isomerism. Draw the structural formula and state the name of the other geometrical isomer.
Identify the structural formula of an isomer of but-2-ene which does not decolourize bromine water, Br2(aq).
(i) Outline two reasons why the polymers of the alkenes are of economic importance.
(ii) State the type of polymerization reaction shown by the alkene in part (a).
(iii) Deduce the structure of the resulting polymer showing three repeating units.
(iv) Explain why monomers are often gases or volatile liquids, but polymers are solids.
Markscheme
(i) (bond formed by) sideways overlap;
(of) p orbitals;
Marks awarded either from sketch or from explanation.
(ii) C(l) is sp3 and C(2) is sp2;
 ;
;
cis but-2-ene/Z-but-2-ene;
 ;
;
(i) synthesis of materials not naturally available/plastics;
chemically unreactive materials produced;
wide range of uses/physical properties / versatile;
cheap;
large industry;
uses a limited natural resource;
Award [2] for any two.
(ii) addition;
(iii) 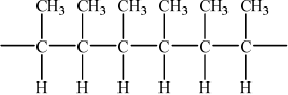 ;
;
Must show continuation bonds.
Ignore bracket around the 6 carbons.
Must have 6 carbons joined to each other along chain.
(iv) monomers are smaller molecules / have smaller surface area than polymers;
Accept monomers have lower molecular mass.
with weaker intermolecular/Van der Waals’/London/dispersion forces;
Accept opposite argument for polymers.
Examiners report
This question was generally well answered and many high scores were seen. Most candidates were able to explain the formation of \(\pi \) bonds in (a) and identify the type of hybridization present.
Many candidates drew structures which were not geometric isomers in (b) with but-1-ene a common incorrect answer.
In (c) only the best candidates were able to identify a cycloalkane as a saturated isomer and it was fairly common to find structures that included double bonds despite the guidance in the question.
The economic importance of addition polymers was well known in (d) with most candidates stating that they were plastics with versatile properties and low cost.
Addition polymerisation was well recalled but a large number of candidates made mistakes with the structure of the polymer. Continuation bonds, for example, were often missing from the ends. Many understood in terms of molecular size, why polymers have higher boiling points than monomers but not all correctly attributed it to the stronger van der Waals forces between the molecules.
But-2-ene belongs to the homologous series of the alkenes.
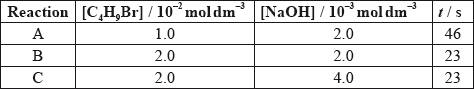
The time taken to produce a certain amount of product using different initial concentrations of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\) and NaOH is measured. The results are shown in the following table.
Outline three features of a homologous series.
Describe a test to distinguish but-2-ene from butane, including what is observed in each case.
2-bromobutane can be produced from but-2-ene. State the equation of this reaction using structural formulas.
State what is meant by the term stereoisomers.
Explain the existence of geometrical isomerism in but-2-ene.
Deduce the order of reaction with respect to \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\) and NaOH, using the data above.
\({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\)
NaOH:
Deduce the rate expression.
Based on the rate expression obtained in (c) (ii) state the units of the rate constant, \(k\).
Halogenalkanes can react with NaOH via \({{\text{S}}_{\text{N}}}{\text{1}}\) and \({{\text{S}}_{\text{N}}}{\text{2}}\) type mechanisms. Explain why \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\) reacts via the mechanism described in (d) (i).
Identify the rate-determining step of this mechanism.
Markscheme
same functional group / same general formula;
difference between successive members is \({\text{C}}{{\text{H}}_{\text{2}}}\);
similar chemical properties;
Do not accept “same” chemical properties.
gradually changing physical properties;
adding bromine (water);
but-2-ene: brown/orange to colourless / decolourizes bromine water and
butane: does not change colour;
OR
adding acidified potassium permanganate solution/KMnO4(aq);
but-2-ene: purple to colourless/brown and
butane: does not change colour;
OR
adding Baeyer’s reagent;
but-2-ene: purple/pink to brown and
butane: does not change colour;
Do not accept “clear” or “transparent” for “colourless”.
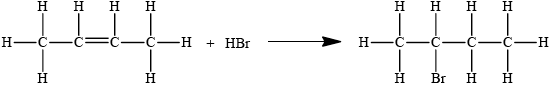
Accept condensed structural formula.
Penalise missing H atoms or incorrect bonds (such as C–HO, C–H2C) once only in the whole paper.
compounds with the same structural formula but different arrangement of atoms (in space);
(but-2-ene exists as) cis-but-2-ene and trans-but-2-ene /
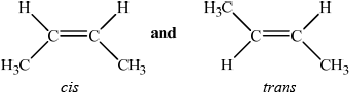 ;
;
restricted rotation of C=C/double bond;
C4H9Br:
[C4H9Br] doubles and time halves/rate doubles/rate proportional to [C4H9Br];
Do not accept rate increases when [C4H9Br] increases.
NaOH:
[NaOH] doubles and time/rate does not change/rate independent of [NaOH];
C4H9Br: first order and NaOH: zero order;
rate \( = k[{{\text{C}}_4}{{\text{H}}_9}{\text{Br}}]\);
Accept ECF.
\({{\text{s}}^{ - 1}}\);
Accept ECF.
greater stability of tertiary carbocation;
steric hindrance for \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism;
positive inductive effect (of alkyl groups);
Do not allow ECF.
the first step / \({\text{B}}{{\text{r}}^ - }\) leaving / formation of carbocation;
Do not allow ECF.
Examiners report
Features of an homologous series need to be learnt; this was answered relatively poorly.
The most common reagent was bromine (some indeed used liquid bromine!) and the common errors were using HBr and describing “colourless” as “clear”.
In (iii), some gave the equation backwards, a consequence, perhaps, of misreading the question.
In (iv) many referred to “same molecular formula” rather than “same structural formula”.
The lack of rotation about the double bond in (v) was not well described.
In (c) (i) the explanations were a little vague, some candidates perhaps being fooled by the data of time rather than rate. Many expected to be given marks for a series of numbers and calculations without explanations.
Answers to (ii) were usually consistent with (i).
Answers to (iii) were usually consistent with (i).
(ii) was rarely answered correctly while the answer to (iii) was patchy.
(ii) was rarely answered correctly while the answer to (iii) was patchy.
Existence of isomers leads to diversity of organic compounds.
(a) Describe what is meant by the term stereoisomers.
(b) 1,3-dichlorocyclobutane exists as geometrical isomers, a form of stereoisomers.
(i) Draw and name the two geometrical isomers of 1,3-dichlorocyclobutane.
(ii) Identify the isomer with the higher boiling point and explain your reasoning.
Markscheme
(a) compounds with same structural formula;
Do not allow “same molecular or chemical formula”.
but different arrangement of atoms in space/spatial arrangement;
(b) (i)
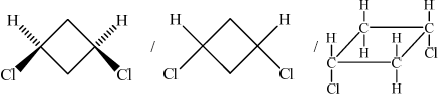 ;
;
Cis(-1,3-dichlorocyclobutane)
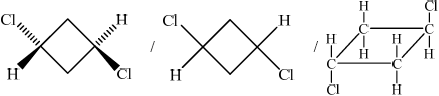 ;
;
Trans(-1,3-dichlorocyclobutane)
Need clear cis/trans structure and name for each mark.
(ii) cis (higher boiling point);
cis (more) polar / trans non-polar/less polar;
cis experiences stronger (permanent) dipole-dipole interaction / trans experiences no/(much) less dipole-dipole interaction;
Do not accept just strong forces without reference to dipole-dipole interaction.
Examiners report
Only a handful of candidates gave the correct definition of the term stereoisomers. Some stated that these had the same chemical or molecular formula with no reference to structural formula. Stereoisomers are compounds with the same structural formula, but with a different arrangement of the atoms in space. A majority of candidates drew correct formulas of the two geometrical isomers of 1,3-dichlorocyclobutane, but some missed the names of the compounds. Even when the notion of cis and trans seemed to be generally understood, the poor representation of molecules proved challenging for some where difference between the 2 isomers drawn was not at all clear.
A few candidates did not realise that the compound was cyclobutane and not straight chain butane. Nomenclature also emerged as a hindrance in the correct grasp of the topic with some candidates showing structures that had little resemblance to the names.
A good number of candidates identified the cis isomer as having the higher boiling point because it is more polar and experiences stronger dipole-dipole interactions between the molecules. Many candidates failed to provide enough details for the type of intermolecular interaction. A number of candidates incorrectly identified the trans as the polar molecule with the higher melting. Quite a few of the weaker candidates used arguments in terms of packing of the molecule and failed to score any mark.
Below are four structural isomers with molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\). State the name of each of the isomers a, b, c and D.
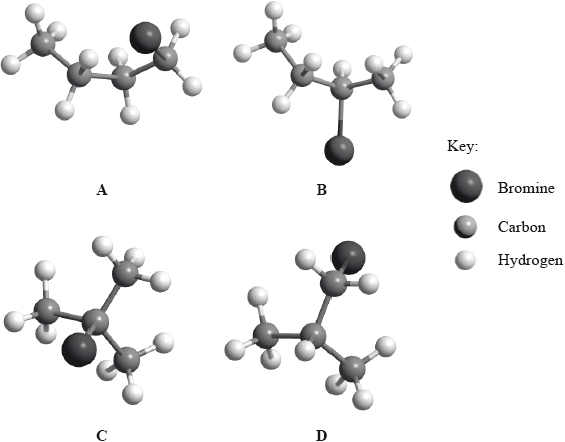
Identify the isomer(s) which will react with aqueous sodium hydroxide almost exclusively by an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. State the meaning of the symbols in the term \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism.
Using the formula RBr to represent a bromoalkane, state an equation for the rate determining step of this \({{\text{S}}_{\text{N}}}{\text{1}}\) reaction.
Identify one isomer that will react with aqueous sodium hydroxide almost exclusively by an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism. Draw the mechanism for this reaction using curly arrows to represent the movement of electron pairs. Include the structural formulas of the transition state and the organic product.
State and explain how the rates of the reactions in parts (b) (i) and (b) (iii) are affected when the concentration of the sodium hydroxide is doubled.
State and explain how the rate of reaction of 1-bromobutane with sodium hydroxide compares with that of 1-chlorobutane with sodium hydroxide.
Identify the isomer of C4H9Br that can exist as stereoisomers. Outline how a polarimeter will distinguish between the isomers, and how their physical and chemical properties compare.
Markscheme
A: l-bromobutane;
B: 2-bromobutane;
C: 2-bromo-2-methylpropane;
D: 1-bromo-2-methylpropane;
Penalize incorrect punctuation, e.g. commas for hyphens, only once.
Accept 2-bromomethylpropane and 1-bromomethylpropane for C and D respectively.
C/2-bromo-2-methylpropane;
unimolecular nucleophilic substitution;
Accept first order in place of unimolecular.
\({\text{RBr}} \to {{\text{R}}^ + } + {\text{B}}{{\text{r}}^ - }\);
Allow use of 2-bromo-2-methylpropane instead of RBr.
A/1-bromobutane/D/1-bromo-2-methylpropane;
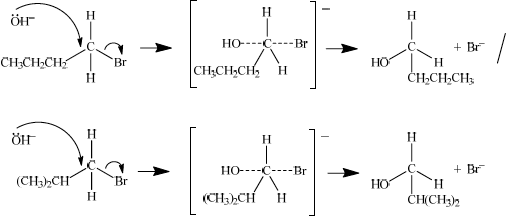
curly arrow going from lone pair/negative charge on O in \({\text{O}}{{\text{H}}^ - }\) to C;
Do not allow curly arrow originating on H in OH–.
curly arrow showing Br leaving;
Accept curly arrow either going from bond between C and Br to Br in 1-bromobutane or in the transition state.
representation of transition state showing negative charge, square brackets and partial bonds;
Do not penalize if HO and Br are not at 180° to each other.
Do not award M4 if OH----C bond is represented.
(b) (i) no change as \({\text{[O}}{{\text{H}}^ - }{\text{]}}\) does not appear in rate equation/in the rate determining step;
(b) (iii) rate doubles as the rate is proportional to \({\text{[O}}{{\text{H}}^ - }{\text{]}}\) / \({\text{O}}{{\text{H}}^ - }\) appears in the ratedetermining/
slow step / first order with respect to \({\text{O}}{{\text{H}}^ - }\);
Award [1] if correctly predicts no rate change for SN1 and doubling of rate for SN2 of without suitable explanation.
rate of 1-bromobutane is faster;
C–Br bond is weaker/breaks more easily than C–Cl bond;
2-bromobutane/B;
(plane-) polarized light shone through;
enantiomers rotate plane of plane-polarized light to left or right/opposite directions (by same amount);
Accept “turn” instead of “rotate” but not “bend/reflect”.
physical properties identical (apart from effect on plane-polarized light);
chemical properties are identical (except with other chiral compounds);
Do not accept “similar” in place of “identical”.
Examiners report
This was the least popular question, but there were some very good answers seen. In parts (a) and (b), most candidates were able to correctly name the organic compounds, and identify which halogenoalkane would react via a SN1 or SN2 reaction.
Many candidates stated first order instead of unimolecular, which although we accepted it in this instance is not correct.
Attempts at the mechanism were generally disappointing though, with errors of incorrectly drawn arrows and faults in the transition state frequently occurring. Also candidates often had an arrow coming from an H in \({\text{O}}{{\text{H}}^ - }\) instead of from a lone pair of electrons on O.
Answers to (c) explaining how \({\text{[O}}{{\text{H}}^ - }{\text{]}}\) effects rate were generally good, however, some only predicted and didn’t explain in terms of the rate limiting step.
Answers to (d) were generally good and only the weakest candidates didn‟t state that bromobutane reacted faster as the C-Br bond was weaker.
Most candidates in (e) knew how enantiomers affected plane-polarized light, but few stated that their properties were identical and many instead suggested they were similar.
Alkenes, alcohols and esters are three families of organic compounds with many commercial uses.
An ester which gives apples their characteristic smell contains C, H and O. When \(3.00 \times {10^{ - 3}}{\text{ g}}\) of this ester were completely combusted, \(6.93 \times {10^{ - 3}}{\text{ g}}\) of \({\text{C}}{{\text{O}}_{\text{2}}}\) and \(2.83 \times {10^{ - 3}}{\text{ g}}\) of \({{\text{H}}_{\text{2}}}{\text{O}}\) were produced.
State what is meant by the term stereoisomers.
Determine the empirical formula of the ester, showing your working.
The molar mass of the ester is \({\text{116.18 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Determine its molecular formula.
2-bromobutane is optically active. Draw the two enantiomers of 2-bromobutane and compare their physical and chemical properties.
Markscheme
compounds with same structural/displayed formula but different arrangements of atoms (in space);
Do not accept different 3D structures.
Do not allow similar instead of same.
Mass of C: \(\frac{{6.93 \times {{10}^{ - 3}}12.01}}{{44.01}} = 1.89 \times {10^{ - 3}}/0.00189{\text{ (g)}}\) and
Mass of H: \(\frac{{2 \times 1.01 \times 2.83 \times {{10}^{ - 3}}}}{{18.02}} = 3.17 \times {10^{ - 4}}/0.000317{\text{ (g)}}\);
Mass of O: \(3.00 \times {10^{ - 3}} - 1.89 \times {10^{ - 3}} - 3.17 \times {10^{ - 4}} = 7.93 \times {10^{ - 4}}/0.000793{\text{ (g)}}\);
\({n_C}:{\text{ }}\frac{{1.89 \times {{10}^{ - 3}}}}{{12.01}} = 1.57 \times {10^{ - 4}}/0.000157{\text{ (mol)}}\) and
\({n_H}:{\text{ }}\frac{{3.17 \times {{10}^{ - 4}}}}{{1.01}} = 3.14 \times {10^{ - 4}}/0.000314{\text{ (mol)}}\) and
\({n_O}:{\text{ }}\frac{{7.93 \times {{10}^{ - 4}}}}{{16.00}} = 4.96 \times {10^{ - 5}}/0.0000496{\text{ (mol)}}\);
Empirical formula \( = {{\text{C}}_3}{{\text{H}}_6}{\text{O}}\);
Allow C19H38O6.
Award [4] for correct final answer if alternative working is used.
Award [1 max] for C3H6O/C19H38O6 without working.
\({{\text{C}}_6}{{\text{H}}_{12}}{{\text{O}}_2}\);
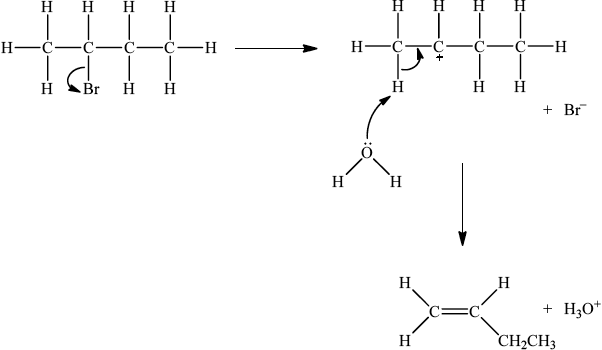
Accept either one of the following two E2 mechanisms:
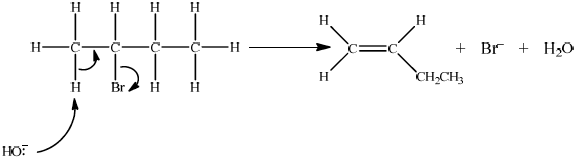
OR
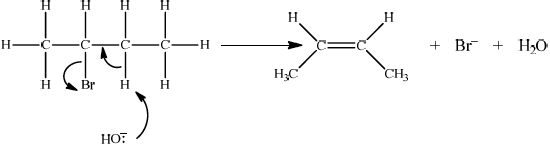
curly arrow going from lone pair/negative charge on O in \({\text{H}}{{\text{O}}^ - }\) to H on \(\beta \)–C;
Do not allow curly arrow originating on H in HO–.
curly arrow going from CH bond to form C=C bond;
curly arrow showing Br leaving;
formation of organic product \({{\text{H}}_2}{\text{C}}\)=\({\text{CH(C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}{\text{)}}/{\text{H(C}}{{\text{H}}_3}{\text{)C}}\)=\({\text{CH(C}}{{\text{H}}_3}{\text{)}}\) and
\({\text{B}}{{\text{r}}^ - }\) and \({{\text{H}}_2}{\text{O}}\);
For this reaction since a strong negatively charged base, HO– is used, resultant mechanism will be E2. However, accept the corresponding E1 mechanism.
If E1, allow the following mechanism:
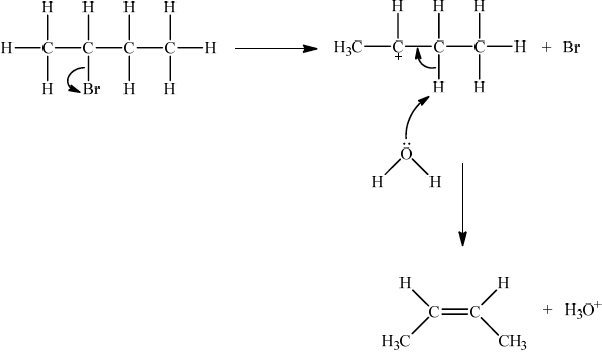
OR
curly arrow showing Br leaving;
representation of secondary carbocation;
curly arrow going from lone pair on O in \({{\text{H}}_2}{\text{O}}\) to H on C adjacent to \({{\text{C}}^ + }\) and curly arrow going from CH bond to form C=C bond;
formation of organic product \({\text{(}}{{\text{H}}_3}{\text{C)CH}}\)=\({\text{CH(C}}{{\text{H}}_3}{\text{)}}\) / \({{\text{H}}_2}{\text{C}}\)=\({\text{CH(C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}{\text{)}}\) and \({\text{B}}{{\text{r}}^ - }\) and \({{\text{H}}_3}{{\text{O}}^ + }\);
For E1 HO– is an alternative to H2O, but if used, H2O forms instead of H3O+.
Examiners report
This was the least popular question in Section B. In part (a) (i), some candidates gave a definition of structural isomers instead of stereoisomers.
Part (b) (i) proved to be very challenging for candidates. A large majority of candidates in fact did not know how to even commence the problem. There were a number of G2 comments all of who stated that it would have been better if the ratios of the amounts of C, H and O were in fact closer to whole number ratios.
In part (ii) of the question the molar mass of the ester was given as \({\text{116.18 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), which meant that taking the experimental data given in (b) (i), the empirical formula is in fact \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{\text{O}}\), with the associated molecular formula of \({{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{{\text{O}}_{\text{2}}}\). The better students realised this and typically gave an answer of \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{\text{O}}\). However, a very small minority did in fact use a scaling factor to suggest an empirical formula of \({{\text{C}}_{{\text{19}}}}{{\text{H}}_{{\text{38}}}}{{\text{O}}_{\text{6}}}\), which was also accepted. In general however for this question, candidates tended to score either scored full marks for parts (i) and (ii), or zero.
In part (iii), some candidates did not show the 3D nature of the two enantiomers which was necessary for M1 and only gave 2D representations. It was encouraging to see a greater percentage of candidates however using tapered (wedge/dash) representations. For M2, many did not mention the fact that the two optical isomers rotate the plane of polarized light in opposite directions. Some did not state plane.
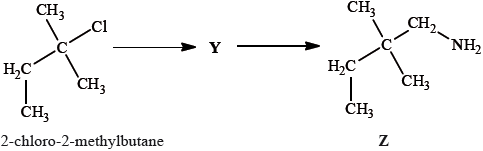
2-methylbutan-2-ol, \({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{C(OH)C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\), is a liquid with a smell of camphor that was formerly used as a sedative. One way of producing it starts with 2-methylbut-2-ene.
As well as 2-methylbutan-2-ol, the reaction also produces a small quantity of an optically active isomer, X.
2-methylbutan-2-ol can also be produced by the hydrolysis of 2-chloro-2-methylbutane, \({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{CCl}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\), with aqueous sodium hydroxide.
2-chloro-2-methylbutane contains some molecules with a molar mass of approximately \({\text{106 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) and some with a molar mass of approximately \({\text{108 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
2-chloro-2-methylbutane can also be converted into compound Z by a two-stage reaction via compound Y:
State the other substances required to convert 2-methylbut-2-ene to 2-methylbutan-2-ol.
Explain whether you would expect 2-methylbutan-2-ol to react with acidified potassium dichromate(VI).
State what is meant by optical activity.
State what optical activity indicates about the structure of the molecule.
Optical activity can be detected using a polarimeter. Explain how this works.
Deduce the structural formula of X.
Explain why 2-methylbut-2-ene is less soluble in water than 2-methylbutan-2-ol.
Explain the mechanism of this reaction using curly arrows to represent the movement of electron pairs.
State the rate expression for this reaction and the units of the rate constant.
Suggest why, for some other halogenoalkanes, this hydrolysis is much more effective in alkaline rather than in neutral conditions.
Outline why there are molecules with different molar masses.
Draw the structure of Y.
State the reagent and any catalyst required for both the formation of Y and the conversion of Y into Z.
Formation of Y:
Conversion of Y into Z:
Markscheme
water/\({{\text{H}}_{\text{2}}}{\text{O}}\);
Accept steam.
(concentrated) sulfuric acid/\({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\) (catalyst);
Accept phosphoric acid/H3PO4.
Award [2] for HBr and NaOH (two-stage process via the halogenoalkane).
not react;
tertiary alcohol (not easily oxidized);
rotates the plane (of polarization) of plane polarized light;
Accept answers in which one of the “plane”s is missing.
two isomers that are enantiomers/chiral/non-superimposable mirror images;
Accept “contains an asymmetric/chiral carbon” or “contains a carbon bonded to four different groups”.
polarizes light / polarized light source;
light passed through sample;
analyser / second polarizer detects whether plane of polarization rotated;
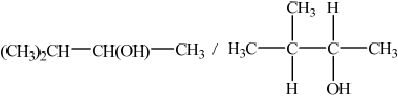 ;
;
Accept C3H7–CH(OH)–CH3, but not CH3–CH2–CH2–CH(OH)–CH3.
2-methylbutan-2-ol has hydroxyl/OH group;
Do not accept “hydroxide group”.
Allow 2-methylbutan-2-ol is an alcohol.
2-methylbutan-2-ol can form H-bonds (to water) / 2-methylbut-2-ene cannot form H-bonds (to water);
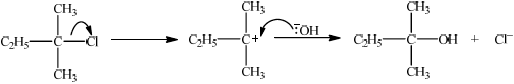
curly arrow showing \({\text{C}}{{\text{l}}^ - }\) leaving;
representation of tertiary carbocation;
curly arrow going from lone pair/negative charge on O in \({\text{H}}{{\text{O}}^ - }\) to \({{\text{C}}^ + }\);
Do not allow arrow originating on H in HO–.
formation of organic product \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C(C}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}{\text{OH}}\) and \({\text{C}}{{\text{l}}^ - }\)/NaCl
(somewhere in mechanism);
Award [3 max] if a candidate gives a fully correct SN2 mechanism.
\({\text{rate}} = {\text{k}} \times \) [2-chloro-2-methylbutane]/\({\text{[C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C(C}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}{\text{Cl]}}\)/[halogenoalkane]
/[R–Cl];
\({{\text{s}}^{ - 1}}\);
hydroxide ion/\({\text{O}}{{\text{H}}^ - }\) is a better nucleophile than water / hydroxide ion/\({\text{O}}{{\text{H}}^ - }\) has negative charge;
undergo \({{\text{S}}_{\text{N}}}{\text{2}}\) hydrolysis / RDS depends on attack of \({\text{O}}{{\text{H}}^ - }\)/hydroxide ion (nucleophile);
Accept other suggestions that are chemically valid.
chlorine can be \(^{{\text{35}}}{\text{Cl}}\)/Cl–35 or \(^{{\text{37}}}{\text{Cl}}\)/Cl–37;
Accept “chlorine can exist as two isotopes”.
Answer must refer to chlorine rather than isotopes in general.
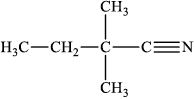 ;
;
Do not accept condensed formulas such as CH3CH2C(CH3)2CN.
Accept the cyanide group as –CN without showing the triple bond.
Formation of Y:
cyanide ion/\({\text{C}}{{\text{N}}^ - }\) / potassium cyanide/KCN;
Accept hydrogen cyanide/HCN.
Conversion of Y into Z:
hydrogen/\({{\text{H}}_{\text{2}}}\);
nickel/Ni / platinum/Pt / palladium/Pd (catalyst);
Examiners report
Many students could recall the reagents for the hydration of an alkene and recognize the alcohol as a tertiary alcohol that would not undergo oxidation. Statements regarding optical activity often lacked precision and betrayed confusion with chirality. Very few could correctly describe how a polarimeter worked, especially the second rotating sheet of polaroid, and students frequently drew the structure of 2-methylbutan-2-ol rather than its chiral isomer. Most students stated that the alcohol was more polar than the alkene, but fewer mentioned that it could form hydrogen bonds to water and even less linked this to the presence of the hydroxyl group. Almost all students recognized that the hydrolysis was \({{\text{S}}_{\text{N}}}{\text{1}}\), with an encouraging number being able to write reasonable mechanisms, though many still lost marks through a lack of precision in where their curly arrows started and ended. Many candidates also stated an appropriate rate equation along with the units of the rate constant. Very few students linked the difference of two molar mass units to the presence in the molecule of chlorine, with its naturally occurring isotopes, and the discussion of any effect on the hydrolysis rate often revealed a lack of clear thinking. In contrast many students correctly identified the nitrile as the intermediate in the chain extension reaction and reagents for its formation and hydrogenation were generally well known.
Many students could recall the reagents for the hydration of an alkene and recognize the alcohol as a tertiary alcohol that would not undergo oxidation. Statements regarding optical activity often lacked precision and betrayed confusion with chirality. Very few could correctly describe how a polarimeter worked, especially the second rotating sheet of polaroid, and students frequently drew the structure of 2-methylbutan-2-ol rather than its chiral isomer. Most students stated that the alcohol was more polar than the alkene, but fewer mentioned that it could form hydrogen bonds to water and even less linked this to the presence of the hydroxyl group. Almost all students recognized that the hydrolysis was \({{\text{S}}_{\text{N}}}{\text{1}}\), with an encouraging number being able to write reasonable mechanisms, though many still lost marks through a lack of precision in where their curly arrows started and ended. Many candidates also stated an appropriate rate equation along with the units of the rate constant. Very few students linked the difference of two molar mass units to the presence in the molecule of chlorine, with its naturally occurring isotopes, and the discussion of any effect on the hydrolysis rate often revealed a lack of clear thinking. In contrast many students correctly identified the nitrile as the intermediate in the chain extension reaction and reagents for its formation and hydrogenation were generally well known.
Many students could recall the reagents for the hydration of an alkene and recognize the alcohol as a tertiary alcohol that would not undergo oxidation. Statements regarding optical activity often lacked precision and betrayed confusion with chirality. Very few could correctly describe how a polarimeter worked, especially the second rotating sheet of polaroid, and students frequently drew the structure of 2-methylbutan-2-ol rather than its chiral isomer. Most students stated that the alcohol was more polar than the alkene, but fewer mentioned that it could form hydrogen bonds to water and even less linked this to the presence of the hydroxyl group. Almost all students recognized that the hydrolysis was \({{\text{S}}_{\text{N}}}{\text{1}}\), with an encouraging number being able to write reasonable mechanisms, though many still lost marks through a lack of precision in where their curly arrows started and ended. Many candidates also stated an appropriate rate equation along with the units of the rate constant. Very few students linked the difference of two molar mass units to the presence in the molecule of chlorine, with its naturally occurring isotopes, and the discussion of any effect on the hydrolysis rate often revealed a lack of clear thinking. In contrast many students correctly identified the nitrile as the intermediate in the chain extension reaction and reagents for its formation and hydrogenation were generally well known.
Many students could recall the reagents for the hydration of an alkene and recognize the alcohol as a tertiary alcohol that would not undergo oxidation. Statements regarding optical activity often lacked precision and betrayed confusion with chirality. Very few could correctly describe how a polarimeter worked, especially the second rotating sheet of polaroid, and students frequently drew the structure of 2-methylbutan-2-ol rather than its chiral isomer. Most students stated that the alcohol was more polar than the alkene, but fewer mentioned that it could form hydrogen bonds to water and even less linked this to the presence of the hydroxyl group. Almost all students recognized that the hydrolysis was \({{\text{S}}_{\text{N}}}{\text{1}}\), with an encouraging number being able to write reasonable mechanisms, though many still lost marks through a lack of precision in where their curly arrows started and ended. Many candidates also stated an appropriate rate equation along with the units of the rate constant. Very few students linked the difference of two molar mass units to the presence in the molecule of chlorine, with its naturally occurring isotopes, and the discussion of any effect on the hydrolysis rate often revealed a lack of clear thinking. In contrast many students correctly identified the nitrile as the intermediate in the chain extension reaction and reagents for its formation and hydrogenation were generally well known.
Many students could recall the reagents for the hydration of an alkene and recognize the alcohol as a tertiary alcohol that would not undergo oxidation. Statements regarding optical activity often lacked precision and betrayed confusion with chirality. Very few could correctly describe how a polarimeter worked, especially the second rotating sheet of polaroid, and students frequently drew the structure of 2-methylbutan-2-ol rather than its chiral isomer. Most students stated that the alcohol was more polar than the alkene, but fewer mentioned that it could form hydrogen bonds to water and even less linked this to the presence of the hydroxyl group. Almost all students recognized that the hydrolysis was \({{\text{S}}_{\text{N}}}{\text{1}}\), with an encouraging number being able to write reasonable mechanisms, though many still lost marks through a lack of precision in where their curly arrows started and ended. Many candidates also stated an appropriate rate equation along with the units of the rate constant. Very few students linked the difference of two molar mass units to the presence in the molecule of chlorine, with its naturally occurring isotopes, and the discussion of any effect on the hydrolysis rate often revealed a lack of clear thinking. In contrast many students correctly identified the nitrile as the intermediate in the chain extension reaction and reagents for its formation and hydrogenation were generally well known.
Many students could recall the reagents for the hydration of an alkene and recognize the alcohol as a tertiary alcohol that would not undergo oxidation. Statements regarding optical activity often lacked precision and betrayed confusion with chirality. Very few could correctly describe how a polarimeter worked, especially the second rotating sheet of polaroid, and students frequently drew the structure of 2-methylbutan-2-ol rather than its chiral isomer. Most students stated that the alcohol was more polar than the alkene, but fewer mentioned that it could form hydrogen bonds to water and even less linked this to the presence of the hydroxyl group. Almost all students recognized that the hydrolysis was \({{\text{S}}_{\text{N}}}{\text{1}}\), with an encouraging number being able to write reasonable mechanisms, though many still lost marks through a lack of precision in where their curly arrows started and ended. Many candidates also stated an appropriate rate equation along with the units of the rate constant. Very few students linked the difference of two molar mass units to the presence in the molecule of chlorine, with its naturally occurring isotopes, and the discussion of any effect on the hydrolysis rate often revealed a lack of clear thinking. In contrast many students correctly identified the nitrile as the intermediate in the chain extension reaction and reagents for its formation and hydrogenation were generally well known.
Many students could recall the reagents for the hydration of an alkene and recognize the alcohol as a tertiary alcohol that would not undergo oxidation. Statements regarding optical activity often lacked precision and betrayed confusion with chirality. Very few could correctly describe how a polarimeter worked, especially the second rotating sheet of polaroid, and students frequently drew the structure of 2-methylbutan-2-ol rather than its chiral isomer. Most students stated that the alcohol was more polar than the alkene, but fewer mentioned that it could form hydrogen bonds to water and even less linked this to the presence of the hydroxyl group. Almost all students recognized that the hydrolysis was \({{\text{S}}_{\text{N}}}{\text{1}}\), with an encouraging number being able to write reasonable mechanisms, though many still lost marks through a lack of precision in where their curly arrows started and ended. Many candidates also stated an appropriate rate equation along with the units of the rate constant. Very few students linked the difference of two molar mass units to the presence in the molecule of chlorine, with its naturally occurring isotopes, and the discussion of any effect on the hydrolysis rate often revealed a lack of clear thinking. In contrast many students correctly identified the nitrile as the intermediate in the chain extension reaction and reagents for its formation and hydrogenation were generally well known.
Many students could recall the reagents for the hydration of an alkene and recognize the alcohol as a tertiary alcohol that would not undergo oxidation. Statements regarding optical activity often lacked precision and betrayed confusion with chirality. Very few could correctly describe how a polarimeter worked, especially the second rotating sheet of polaroid, and students frequently drew the structure of 2-methylbutan-2-ol rather than its chiral isomer. Most students stated that the alcohol was more polar than the alkene, but fewer mentioned that it could form hydrogen bonds to water and even less linked this to the presence of the hydroxyl group. Almost all students recognized that the hydrolysis was \({{\text{S}}_{\text{N}}}{\text{1}}\), with an encouraging number being able to write reasonable mechanisms, though many still lost marks through a lack of precision in where their curly arrows started and ended. Many candidates also stated an appropriate rate equation along with the units of the rate constant. Very few students linked the difference of two molar mass units to the presence in the molecule of chlorine, with its naturally occurring isotopes, and the discussion of any effect on the hydrolysis rate often revealed a lack of clear thinking. In contrast many students correctly identified the nitrile as the intermediate in the chain extension reaction and reagents for its formation and hydrogenation were generally well known.
Many students could recall the reagents for the hydration of an alkene and recognize the alcohol as a tertiary alcohol that would not undergo oxidation. Statements regarding optical activity often lacked precision and betrayed confusion with chirality. Very few could correctly describe how a polarimeter worked, especially the second rotating sheet of polaroid, and students frequently drew the structure of 2-methylbutan-2-ol rather than its chiral isomer. Most students stated that the alcohol was more polar than the alkene, but fewer mentioned that it could form hydrogen bonds to water and even less linked this to the presence of the hydroxyl group. Almost all students recognized that the hydrolysis was \({{\text{S}}_{\text{N}}}{\text{1}}\), with an encouraging number being able to write reasonable mechanisms, though many still lost marks through a lack of precision in where their curly arrows started and ended. Many candidates also stated an appropriate rate equation along with the units of the rate constant. Very few students linked the difference of two molar mass units to the presence in the molecule of chlorine, with its naturally occurring isotopes, and the discussion of any effect on the hydrolysis rate often revealed a lack of clear thinking. In contrast many students correctly identified the nitrile as the intermediate in the chain extension reaction and reagents for its formation and hydrogenation were generally well known.
Many students could recall the reagents for the hydration of an alkene and recognize the alcohol as a tertiary alcohol that would not undergo oxidation. Statements regarding optical activity often lacked precision and betrayed confusion with chirality. Very few could correctly describe how a polarimeter worked, especially the second rotating sheet of polaroid, and students frequently drew the structure of 2-methylbutan-2-ol rather than its chiral isomer. Most students stated that the alcohol was more polar than the alkene, but fewer mentioned that it could form hydrogen bonds to water and even less linked this to the presence of the hydroxyl group. Almost all students recognized that the hydrolysis was \({{\text{S}}_{\text{N}}}{\text{1}}\), with an encouraging number being able to write reasonable mechanisms, though many still lost marks through a lack of precision in where their curly arrows started and ended. Many candidates also stated an appropriate rate equation along with the units of the rate constant. Very few students linked the difference of two molar mass units to the presence in the molecule of chlorine, with its naturally occurring isotopes, and the discussion of any effect on the hydrolysis rate often revealed a lack of clear thinking. In contrast many students correctly identified the nitrile as the intermediate in the chain extension reaction and reagents for its formation and hydrogenation were generally well known.
Many students could recall the reagents for the hydration of an alkene and recognize the alcohol as a tertiary alcohol that would not undergo oxidation. Statements regarding optical activity often lacked precision and betrayed confusion with chirality. Very few could correctly describe how a polarimeter worked, especially the second rotating sheet of polaroid, and students frequently drew the structure of 2-methylbutan-2-ol rather than its chiral isomer. Most students stated that the alcohol was more polar than the alkene, but fewer mentioned that it could form hydrogen bonds to water and even less linked this to the presence of the hydroxyl group. Almost all students recognized that the hydrolysis was \({{\text{S}}_{\text{N}}}{\text{1}}\), with an encouraging number being able to write reasonable mechanisms, though many still lost marks through a lack of precision in where their curly arrows started and ended. Many candidates also stated an appropriate rate equation along with the units of the rate constant. Very few students linked the difference of two molar mass units to the presence in the molecule of chlorine, with its naturally occurring isotopes, and the discussion of any effect on the hydrolysis rate often revealed a lack of clear thinking. In contrast many students correctly identified the nitrile as the intermediate in the chain extension reaction and reagents for its formation and hydrogenation were generally well known.
Many students could recall the reagents for the hydration of an alkene and recognize the alcohol as a tertiary alcohol that would not undergo oxidation. Statements regarding optical activity often lacked precision and betrayed confusion with chirality. Very few could correctly describe how a polarimeter worked, especially the second rotating sheet of polaroid, and students frequently drew the structure of 2-methylbutan-2-ol rather than its chiral isomer. Most students stated that the alcohol was more polar than the alkene, but fewer mentioned that it could form hydrogen bonds to water and even less linked this to the presence of the hydroxyl group. Almost all students recognized that the hydrolysis was \({{\text{S}}_{\text{N}}}{\text{1}}\), with an encouraging number being able to write reasonable mechanisms, though many still lost marks through a lack of precision in where their curly arrows started and ended. Many candidates also stated an appropriate rate equation along with the units of the rate constant. Very few students linked the difference of two molar mass units to the presence in the molecule of chlorine, with its naturally occurring isotopes, and the discussion of any effect on the hydrolysis rate often revealed a lack of clear thinking. In contrast many students correctly identified the nitrile as the intermediate in the chain extension reaction and reagents for its formation and hydrogenation were generally well known.
Many students could recall the reagents for the hydration of an alkene and recognize the alcohol as a tertiary alcohol that would not undergo oxidation. Statements regarding optical activity often lacked precision and betrayed confusion with chirality. Very few could correctly describe how a polarimeter worked, especially the second rotating sheet of polaroid, and students frequently drew the structure of 2-methylbutan-2-ol rather than its chiral isomer. Most students stated that the alcohol was more polar than the alkene, but fewer mentioned that it could form hydrogen bonds to water and even less linked this to the presence of the hydroxyl group. Almost all students recognized that the hydrolysis was \({{\text{S}}_{\text{N}}}{\text{1}}\), with an encouraging number being able to write reasonable mechanisms, though many still lost marks through a lack of precision in where their curly arrows started and ended. Many candidates also stated an appropriate rate equation along with the units of the rate constant. Very few students linked the difference of two molar mass units to the presence in the molecule of chlorine, with its naturally occurring isotopes, and the discussion of any effect on the hydrolysis rate often revealed a lack of clear thinking. In contrast many students correctly identified the nitrile as the intermediate in the chain extension reaction and reagents for its formation and hydrogenation were generally well known.
One structural isomer of C4H9Br is a chiral molecule.
Draw the three-dimensional shape of each enantiomer of this isomer showing their spatial relationship to each other.
When one enantiomer undergoes substitution by alkaline hydrolysis approximately 75 % of the product molecules show inversion of configuration. Comment on the mechanisms that occur.
Suggest why the rate of alkaline hydrolysis of an enantiomer of iodopropane is greater than that of an enantiomer of bromopropane.
Markscheme
correct isomer
mirror image shown clearly
SN2 would give inversion of configuration «almost 100%»
OR
SN1 would give «approximately» 50% of each
so mechanism is a mixture of both mechanisms
C–I bond «longer, so» weaker «than C–Br bond»
OR
I– is a better leaving group than Br–
Examiners report
In some countries, ethanol is mixed with gasoline (petrol) to produce a fuel for cars called gasohol.
Deduce a two-step synthesis for each of the following conversions. For each step, state the structural formulas of all reactants and products and state the conditions used in the reactions.
Ethanol to ethyl ethanoate.
Propene to propanone.
The reagents used in an elimination reaction are shown below.
Explain the mechanism of this reaction using curly arrows to represent the movement of electron pairs.
Describe geometrical isomerism.
Draw the geometrical isomers of but-2-ene.
Draw the two enantiomers of butan-2-ol.
Markscheme
\[{\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}}\xrightarrow[{{{\text{H}}^ + }}]{{{{\text{K}}_2}{\text{C}}{{\text{r}}_2}{{\text{O}}_7}}}{\text{C}}{{\text{H}}_3}{\text{COOH}}\xrightarrow[{{{\text{H}}_2}{\text{S}}{{\text{O}}_4}}]{{{\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}}}}{\text{C}}{{\text{H}}_3}{\text{C}}{{\text{O}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3} + {{\text{H}}_2}{\text{O}}\]
Structural formulas of reactants and products
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\) and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH/C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{O}}_{\text{2}}}{\text{H}}\) and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}{\text{ (}} + {{\text{H}}_{\text{2}}}{\text{O)}}\);
Conditions/reagents used
reflux with named suitable acidified oxidising agent and then heat with alcohol and sulfuric acid;
Suitable oxidising agents are potassium dichromate/K2Cr2O7 / sodium dichromate/Na2Cr2O7 / dichromate/Cr2O72– / potassium manganate(VII)/potassium permanganate/KMnO4 / permanganate/manganate (VII)/MnO4–.
Accept H+/H2SO4 instead of sulfuric acid and acidified.
Award [1] for structural formulas of reactants and products and [1] for the correct conditions/reagents used.
\({{\text{H}}_{\text{2}}}{\text{C=CH(C}}{{\text{H}}_{\text{3}}}{\text{)}}\xrightarrow[{{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}{\text{(conc.)}}}]{{{{\text{H}}_{\text{2}}}{\text{O}}}}{\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(OH)C}}{{\text{H}}_{\text{3}}}\xrightarrow[{{{\text{H}}^ + }}]{{{{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}}}{{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{CO}}\)
Structural formulas of reactants and products
\({{\text{H}}_{\text{2}}}{\text{C=CH(C}}{{\text{H}}_{\text{3}}}{\text{)}}\) and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(OH)C}}{{\text{H}}_{\text{3}}}\) and \({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{CO}}\);
Conditions/reagents used
water/\({{\text{H}}_{\text{2}}}{\text{O}}\) and sulfuric acid/\({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\) / dilute acid medium and heat/reflux with suitable acidified oxidising agent;
Suitable oxidising agents are potassium dichromate/K2Cr2O7 / sodium dichromate/Na2Cr2O7 / dichromate/Cr2O72– / potassium manganate(VII)/potassium permanganate/KMnO4 / permanganate/manganate (VII)/MnO4–.
Accept H+/H2SO4 instead of acidified.
Note: If primary alcohol is given as product of first step, and everything else correct, award [1 max].
Accept either full or condensed structural formulas throughout (b).
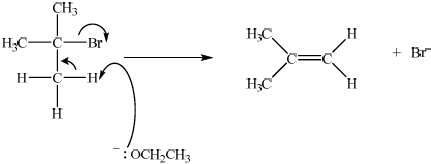
curly arrow going from O of \(^ - {\text{OC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}\) attacking hydrogen;
Allow the curly arrow to originate from either the lone pair or O of –OCH2CH3 but not from H of –OCH2CH3.
Do not award first mark if curly arrow originates from O of NaOCH2CH3.
curly arrow going from the C–H bond on the \(\beta \) carbon to the bond joining the \(\alpha \) carbon to the \(\beta \) carbon and curly arrow showing Br acting as leaving group;
formation of \({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{C=C}}{{\text{H}}_{\text{2}}}\) and \({\text{B}}{{\text{r}}^ - }\);
Allow formation of NaBr for third marking point, if NaOCH2CH3 was used (incorrectly) in the mechanism. Use of NaOCH2CH3 with curly arrow originating on O of NaOCH2CH3 is penalized already in the first marking point.
Accept alternative E1 type mechanism
curly arrow showing Br acting as leaving group to form carbocation;
curly arrow going from O of \(^ - {\text{OC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\) attacking hydrogen;
formation of \({({\text{C}}{{\text{H}}_{\text{3}}})_{\text{2}}}{\text{C=C}}{{\text{H}}_{\text{2}}}\) and Br–;
No marks awarded if a substitution mechanism is given.
compounds with the same (molecular formula and) structural formula but different arrangements of atoms in space / OWTTE;
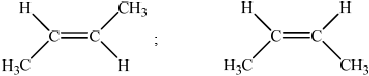 ;
;
Allow [1 max] if structures are correct but arrangement of groups in space does not clearly show the cis/ trans isomerism.
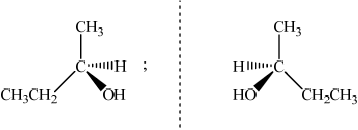 ;
;
Allow [1 max] if the structures are correct but it is not clear that they are mirror images.
Examiners report
In (b) (i) and (ii) only a few candidates answered both questions correctly. Fewer candidates scored one mark in one or both by correctly presenting the structural formulas. Conditions and reagents were in general poorly known.
In (b) (i) and (ii) only a few candidates answered both questions correctly. Fewer candidates scored one mark in one or both by correctly presenting the structural formulas. Conditions and reagents were in general poorly known.
Almost nobody answered (c) correctly. Candidates identified this as an SN1 mechanism. There were a number of G2 comments on this, and one respondent expressed surprise that sodium ethoxide was used as a reagent. However, the candidates were clearly told in the question that the reaction was an elimination reaction and hence should have been able to write the mechanism, as outlined in AS 20.3.2.
Part (d) (i), (ii) and (iii) were all well answered questions but some candidates lost a mark in (iii) because the structures were not represented as clear mirror images of each other.
Part (d) (i), (ii) and (iii) were all well answered questions but some candidates lost a mark in (iii) because the structures were not represented as clear mirror images of each other.
Part (d) (i), (ii) and (iii) were all well answered questions but some candidates lost a mark in (iii) because the structures were not represented as clear mirror images of each other.
The compound \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{7}}}{\text{Cl}}\) can exhibit stereoisomerism.
The reaction between bromoethane, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Br}}\), and potassium cyanide is an example of a nucleophilic substitution reaction.
Draw the structural formulas of the two geometrical isomers of 1-chloro-but-2-ene.
Explain why 1-chloro-but-2-ene shows geometrical isomerism.
Draw the structural formula of one isomer of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{7}}}{\text{Cl}}\) that shows optical isomerism and identify the chiral carbon atom with an asterisk (*).
State whether this reaction is SN1 or SN2.
Explain the mechanism of the reaction using curly arrows to represent the movement of electron pairs.
The organic product obtained in part (c) (ii) can be reduced to form an amine. State an equation for the reaction, naming the catalyst involved.
Markscheme
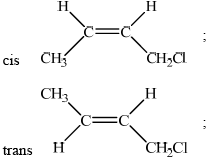
no rotation possible due to double bond/pi bond;
Accept hindered or restricted rotation.
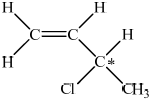
correct structural formula;
chiral carbon atom identified;
SN2;
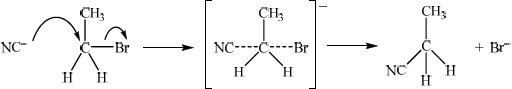
curly arrow going from \({\text{C}}{{\text{N}}^ - }\) to C;
curly arrow showing Br leaving;
Curly arrow may be represented on transition state.
representation of transition state, showing negative charge and dotted lines;
products;
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CN}} + {\text{2}}{{\text{H}}_{\text{2}}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{N}}{{\text{H}}_{\text{2}}}\);
Ni / Pt / Pd;
Examiners report
The standard of organic chemistry this session was slightly better when compared to previous sessions. In part (a), some candidates missed that no rotation is possible due to the pi bond. The question required candidates to draw the two geometrical isomers of 1-chloro-but-2-ene but some candidates had drawn the isomers of 1-chloro-but-1-ene or 2-chloro-but-2-ene. Only the able candidates could draw the optical isomer of C4H7Cl and identify the chiral carbon atom.
In part (c), the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism between bromoethane and potassium cyanide proved to be a challenge as candidates continued to make the same errors as found in previous sessions, such as an incorrect placement of curly arrows, the omission of non-bonding pairs of electrons on the nucleophile and the failure to include partial bonds and an overall charge in the formula of the \({{\text{S}}_{\text{N}}}{\text{2}}\) transition state. Candidates are encouraged to show the entering and leaving groups at 180° instead of 90° on the transition state.
In part (c), the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism between bromoethane and potassium cyanide proved to be a challenge as candidates continued to make the same errors as found in previous sessions, such as an incorrect placement of curly arrows, the omission of non-bonding pairs of electrons on the nucleophile and the failure to include partial bonds and an overall charge in the formula of the \({{\text{S}}_{\text{N}}}{\text{2}}\) transition state. Candidates are encouraged to show the entering and leaving groups at 180° instead of 90° on the transition state.
In part (c), the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism between bromoethane and potassium cyanide proved to be a challenge as candidates continued to make the same errors as found in previous sessions, such as an incorrect placement of curly arrows, the omission of non-bonding pairs of electrons on the nucleophile and the failure to include partial bonds and an overall charge in the formula of the \({{\text{S}}_{\text{N}}}{\text{2}}\) transition state. Candidates are encouraged to show the entering and leaving groups at 180° instead of 90° on the transition state.
But-1-ene and 1-aminobutane (1-butylamine) can both be prepared from 1-bromobutane.
2-bromobutane and 2-bromo-2-methylpropane are two isomers of 1-bromobutane.
Identify the type of reaction and explain the mechanism for the preparation of but-1-ene from 1-bromobutane using curly arrows to represent the movement of electron pairs.
State the equation (using structural formulas) for the preparation of 1-aminobutane from 1-bromobutane. State the necessary reagents and conditions of the reaction.
Explain the mechanism for the preparation of 1-aminobutane from 1-bromobutane using curly arrows to represent the movement of electron pairs.
Draw the structures of the two mirror images of the isomer that can exhibit optical isomerism.
Describe how the two optical isomers can be distinguished practically using plane-polarized light.
Explain why the mechanism of the reaction will be different if 1-bromobutane is replaced by 2-bromo-2-methylpropane to form 2-amino-2-methylpropane in the reaction in part (a) (iv).
Markscheme
elimination reaction ;
Then accept either E1 or E2 mechanism.
E1
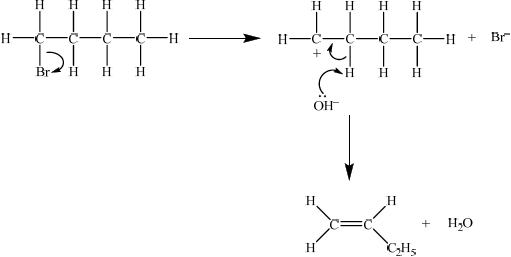
curly arrow showing bromine leaving the halogenoalkane;
\({\text{O}}{{\text{H}}^ - }\) acting as base on the intermediate carbocation;
E2
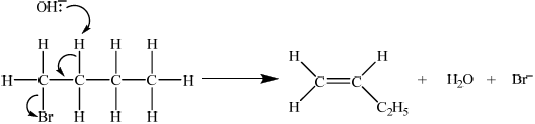
curly arrow showing\({\text{O}}{{\text{H}}^ - }\) acting as base on H bonded to C;
concerted curly arrows showing Br leaving C–Br;
\({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{Br}} + {\text{N}}{{\text{H}}_3} \to {\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{N}}{{\text{H}}_2} + {\text{HBr}}\);
ammonia/\({\text{N}}{{\text{H}}_{\text{3}}}\);
warm / excess ammonia (to prevent secondary amines etc.);
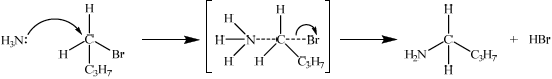
curly arrow from ammonia (to form transition state);
correct transition state;
curly arrow from bond to Br atom in either the first or second step;
formation of HBr and organic product;
Accept a second molecule of NH3 removing H+ from the transition state to give NH4+ and Br– as products.
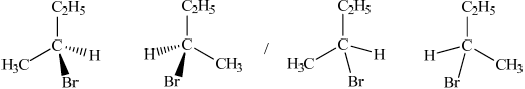
Award [1] for correct structure and [1] for correct 3-D representation of both enantiomers.
polarimeter (to measure angle of rotation);
the plane of plane-polarized light rotates in opposite directions (by the different enantiomers);
2-bromo-2-methylpropane is tertiary / 1-bromobutane is primary;
2-bromo-2-methylpropane goes by SN1 / 1-bromobutane by \({{\text{S}}_{\text{N}}}{\text{2}}\);
intermediate carbocation more stable for tertiary;
no space around tertiary carbon for five groups (in \({{\text{S}}_{\text{N}}}{\text{2}}\) transition state);
Examiners report
This question was the least popular of the Section B questions. Some candidates were very well prepared and scored well, while many struggled to write correct mechanisms with curly arrows in the right place. For a small number of candidates, all parts of the question other than the identification of a functional group proved very difficult. Additionally, in part (a), few candidates knew the details of the reagents and conditions for a range of reaction types.
This question was the least popular of the Section B questions. Some candidates were very well prepared and scored well, while many struggled to write correct mechanisms with curly arrows in the right place. For a small number of candidates, all parts of the question other than the identification of a functional group proved very difficult. Additionally, in part (a), few candidates knew the details of the reagents and conditions for a range of reaction types.
This question was the least popular of the Section B questions. Some candidates were very well prepared and scored well, while many struggled to write correct mechanisms with curly arrows in the right place. For a small number of candidates, all parts of the question other than the identification of a functional group proved very difficult. Additionally, in part (a), few candidates knew the details of the reagents and conditions for a range of reaction types.
In part (b), very few candidates could draw three-dimensional structures of optical isomers, although many gained a mark for correctly identifying the structure which could have enantiomers.
Few mentioned a polarimeter to distinguish between the two optical isomers, although several described in clear detail a practical method to do so.
Consider the two-stage reaction pathway below.
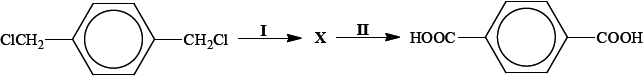
Deduce the structural formula of compound X.
State the reagents and conditions required for stage II of the pathway.
Reagents:
Conditions:
Markscheme
 ;
;
Accept left hand end written as CH2(OH)–.
Reagents: acidified/H\(^ + \) dichromate ion/\({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_7^{2 - }\)/potassium dichromate/\({{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}\)/sodium dichromate/\({\text{N}}{{\text{a}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}\);
Accept acidified/H+ permanganate ion/MnO4–/potassium permanganate/KMnO4.
Conditions: reflux;
Accept “heat” (as the intermediate aldehyde will not be volatile enough to vaporize significantly) unless distillation mentioned.
Examiners report
This was frequently well answered with many students gaining good marks. A common mistake was for students to identify the bond present in the polymer rather than the type of polymerization.
This was frequently well answered with many students gaining good marks. A common mistake was for students to identify the bond present in the polymer rather than the type of polymerization.
Consider the following reactions.
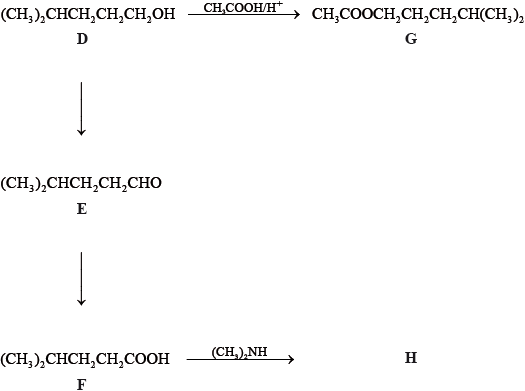
State the IUPAC names of each of the compounds, D, E, F and G.
D:
E:
F:
G:
State the reagents and reaction conditions used to convert D to E and D to F directly.
Discuss the volatility of E compared to F.
Markscheme
D: 4-methylpentan-1-ol;
Allow 4-methyl-1-pentanol.
E: 4-methylpentanal;
F: 4-methylpentanoic acid;
G: 4-methylpentyl ethanoate;
Allow 4-methylpentyl acetate.
Award [2] for all four correct, [1 max] for two or three correct.
Award [1 max] if all suffices correct but prefix (4-methyl or pent) not correct.
For both reactions reagents:
named suitable acidified oxidizing agent;
Suitable oxidizing agents are potassium dichromate(VI)/K2Cr2O7 / sodium dichromate(VI)/Na2Cr2O7 / dichromate/Cr2O72– / potassium manganate(VII)/potassium permanganate/KMnO4 / permanganate/manganate(VII)/MnO4–.
Accept H+/H2SO4 instead of sulfuric acid and acidified.
Allow potassium dichromate or sodium dichromate (i.e. without (VI)) or potassium manganate (i.e. without (VII).
Conditions:
distillation for D to E and reflux for D to F;
Award [1 max] if correct reagents and conditions identified for one process only.
Volatility:
E more volatile than F;
hydrogen bonding in carboxylic acid/F;
Accept converse argument.
Examiners report
Many candidates only scored one mark.
Distillation often was not mentioned.
(iv) was very well answered.
Some reactions of but-2-ene are given below.
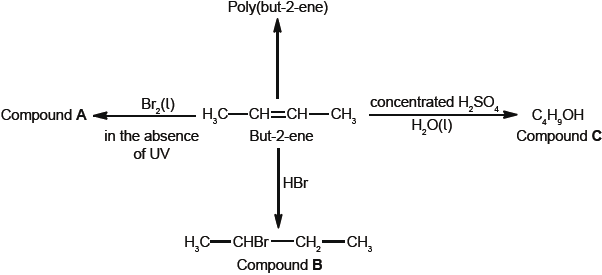
But-2-ene can exist as two geometrical isomers. Cis-trans is a form of stereoisomerism.
Deduce the full structural formula of compound A.
Apply IUPAC rules to name compound A.
Describe the colour change observed when excess but-2-ene reacts with bromine to form compound A.
(i) Outline two reasons why the polymerization of alkenes is of economic importance.
(ii) Identify the structure of the repeating unit of poly(but-2-ene).
Compound C, \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\), can also be formed by reacting compound B, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHBrC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\), with aqueous potassium hydroxide. This reaction proceeds by both \({{\text{S}}_{\text{N}}}{\text{1}}\) and \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanisms. Explain the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism, using curly arrows to represent the movement of electron pairs.
Explain why the hydroxide ion is a better nucleophile than water.
Compound C, \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\), can be oxidized by acidified potassium dichromate(VI) to form compound F.
(i) State the name of the functional group present in compound F.
(ii) Deduce the structural formula of an alcohol which is a structural isomer of compound C and cannot be oxidized by acidified potassium dichromate(VI).
Explain why but-2-ene is more volatile than compound C.
Deduce the equation for the complete combustion of compound C.
Define the term stereoisomers.
State the conditions needed for a compound to show cis-trans.
Draw the structures of the two geometrical isomers of but-2-ene, clearly identifying each as cis or trans.
Markscheme
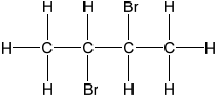 ;
;
Accept bromine atoms cis to each other.
2,3-dibromobutane;
Do not penalize the incorrect use of spaces, comma or hyphen.
red/brown/orange/yellow to colourless/decolourized;
Do not accept clear.
Do not accept just “decolorized”.
(i) (synthesis of) plastics/polymers/organic materials not naturally available / synthetic materials;
wide range of uses/physical properties / versatile;
large industry / many tons of plastics consumed by society / OWTTE;
Do not accept “useful” for M2.
Award [1 max] if specific addition polymer and its use is given.
Penalize reference to condensation polymers once only.
(ii)  ;
;
Ignore n.
Brackets are not required for the mark, but continuation bonds are.
Do not penalize if methyl groups are trans to each other.
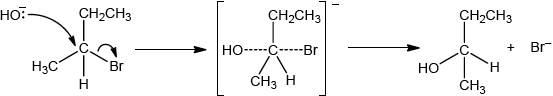
curly arrow going from lone pair/negative charge on O in \({\text{H}}{{\text{O}}^ - }\) to C;
Do not accept curly arrow originating on H in \(H{O^ - }\).
curly arrow showing Br leaving;
Accept curly arrow either going from bond between C and Br to Br in 2-bromobutane or in the transition state.
Accept if arrow goes from C–Br bond to/or beyond Br.
representation of transition state showing negative charge, square brackets and partial bonds;
Do not penalize if HO and Br are not at 180° to each other.
Do not award M3 if OH----C bond is represented.
formation of organic product \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHOHC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\) and \({\text{KBr/B}}{{\text{r}}^ - }\);
\({\text{O}}{{\text{H}}^ - }\) has a negative charge/higher electron density;
stronger attraction to the carbon atom with the partial positive charge / OWTTE;
Do not accept just stronger attraction.
Reference to carbon atom needed for M2.
(i) carbonyl;
Accept ketone.
(ii) 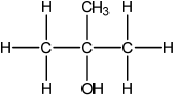 ;
;
Accept condensed or full structural formula.
hydrogen bonding in compound C;
dipole-dipole forces in C / C is more polar;
C has greater molar mass/more dispersion/London/instantaneous induced dipole-induced dipole forces/van der Waal forces;
Accept converse argument.
Award [1 max] for stronger intermolecular forces.
\({{\text{C}}_4}{{\text{H}}_9}{\text{OH(l)}} + {\text{6}}{{\text{O}}_2}{\text{(g)}} \to {\text{4C}}{{\text{O}}_2}{\text{(g)}} + {\text{5}}{{\text{H}}_{\text{2}}}{\text{O(l)}}\);
Ignore state symbols.
compounds with the same structural formula and different arrangement in space/3D structures;
Accept molecular formula instead of structural formula.
Do not accept “similar” instead of “same”.
restricted rotation around a (double) bond;
carbon atoms of the C=C/carbon-carbon double bond (in alkene)/carbon atoms of the C–C/carbon-carbon single bond (in cycloalkane) must have two different atoms/groups of atoms / OWTTE;
Do not accept “functional groups” for “groups of atoms” in M2.

Award [1 max] if cis and trans isomers are correctly drawn and identified for alkene other than but-2-ene.
Award [1 max] if student draws and labels one structure correctly but not the other.
Examiners report
Most candidates were able to give the full structural formula but marks were lost by some as they gave the condensed formula rather than the full structural formula as demanded by the question. Most were able to apply IUPAC rules and name A but some omitted the “di” from dibromobutane. The colour change observed when but-2-ene reacts with bromine was well known, but knowledge of the economic importance of the polymerisation of alkenes was limited with many candidates restricting their answers to identifying specific plastics such a polythene. Many responses included incorrect references to nylon and margarine. Most candidates were able to identify the repeating unit of poly(but-2-ene). The explanation of the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism was more successful than in previous sessions although a common error was a curly arrow originating from the hydrogen atom in the hydroxide ion rather than the oxygen. Most candidates were able to explain the higher reactivity of the hydroxide ion compared to the water molecule in terms of charge but only a minority referred to the attraction between the nucleophile and low electron density of the carbon atom. The naming of 2-methylbutanenitrile was generally well done although small errors were accepted and the reagents needed for the hydrogenation of 2-methylbutanenitrile were also generally known. A number of candidates omitted the branching methyl group in the amide formed with ethanoic acid and confused aldehydes with ketone and only a small minority referred to the carbonyl group. Most candidates identified only hydrogen bonds in compound C and did not refer to the dipole-dipole forces or van der Waals’ forces also present or explicitly compare the relative strength of the different intermolecular forces in the two molecules. Some incorrectly referred to covalent bonding in their explanation. The equation for the complete combustion of compound C was generally well known. The term stereoisomer was well understood but many candidates did not refer to the restricted rotation around a double bond. Most candidates were able draw the structures of cis and trans but-2-ene.
Most candidates were able to give the full structural formula but marks were lost by some as they gave the condensed formula rather than the full structural formula as demanded by the question. Most were able to apply IUPAC rules and name A but some omitted the “di” from dibromobutane. The colour change observed when but-2-ene reacts with bromine was well known, but knowledge of the economic importance of the polymerisation of alkenes was limited with many candidates restricting their answers to identifying specific plastics such a polythene. Many responses included incorrect references to nylon and margarine. Most candidates were able to identify the repeating unit of poly(but-2-ene). The explanation of the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism was more successful than in previous sessions although a common error was a curly arrow originating from the hydrogen atom in the hydroxide ion rather than the oxygen. Most candidates were able to explain the higher reactivity of the hydroxide ion compared to the water molecule in terms of charge but only a minority referred to the attraction between the nucleophile and low electron density of the carbon atom. The naming of 2-methylbutanenitrile was generally well done although small errors were accepted and the reagents needed for the hydrogenation of 2-methylbutanenitrile were also generally known. A number of candidates omitted the branching methyl group in the amide formed with ethanoic acid and confused aldehydes with ketone and only a small minority referred to the carbonyl group. Most candidates identified only hydrogen bonds in compound C and did not refer to the dipole-dipole forces or van der Waals’ forces also present or explicitly compare the relative strength of the different intermolecular forces in the two molecules. Some incorrectly referred to covalent bonding in their explanation. The equation for the complete combustion of compound C was generally well known. The term stereoisomer was well understood but many candidates did not refer to the restricted rotation around a double bond. Most candidates were able draw the structures of cis and trans but-2-ene.
Most candidates were able to give the full structural formula but marks were lost by some as they gave the condensed formula rather than the full structural formula as demanded by the question. Most were able to apply IUPAC rules and name A but some omitted the “di” from dibromobutane. The colour change observed when but-2-ene reacts with bromine was well known, but knowledge of the economic importance of the polymerisation of alkenes was limited with many candidates restricting their answers to identifying specific plastics such a polythene. Many responses included incorrect references to nylon and margarine. Most candidates were able to identify the repeating unit of poly(but-2-ene). The explanation of the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism was more successful than in previous sessions although a common error was a curly arrow originating from the hydrogen atom in the hydroxide ion rather than the oxygen. Most candidates were able to explain the higher reactivity of the hydroxide ion compared to the water molecule in terms of charge but only a minority referred to the attraction between the nucleophile and low electron density of the carbon atom. The naming of 2-methylbutanenitrile was generally well done although small errors were accepted and the reagents needed for the hydrogenation of 2-methylbutanenitrile were also generally known. A number of candidates omitted the branching methyl group in the amide formed with ethanoic acid and confused aldehydes with ketone and only a small minority referred to the carbonyl group. Most candidates identified only hydrogen bonds in compound C and did not refer to the dipole-dipole forces or van der Waals’ forces also present or explicitly compare the relative strength of the different intermolecular forces in the two molecules. Some incorrectly referred to covalent bonding in their explanation. The equation for the complete combustion of compound C was generally well known. The term stereoisomer was well understood but many candidates did not refer to the restricted rotation around a double bond. Most candidates were able draw the structures of cis and trans but-2-ene.
Most candidates were able to give the full structural formula but marks were lost by some as they gave the condensed formula rather than the full structural formula as demanded by the question. Most were able to apply IUPAC rules and name A but some omitted the “di” from dibromobutane. The colour change observed when but-2-ene reacts with bromine was well known, but knowledge of the economic importance of the polymerisation of alkenes was limited with many candidates restricting their answers to identifying specific plastics such a polythene. Many responses included incorrect references to nylon and margarine. Most candidates were able to identify the repeating unit of poly(but-2-ene). The explanation of the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism was more successful than in previous sessions although a common error was a curly arrow originating from the hydrogen atom in the hydroxide ion rather than the oxygen. Most candidates were able to explain the higher reactivity of the hydroxide ion compared to the water molecule in terms of charge but only a minority referred to the attraction between the nucleophile and low electron density of the carbon atom. The naming of 2-methylbutanenitrile was generally well done although small errors were accepted and the reagents needed for the hydrogenation of 2-methylbutanenitrile were also generally known. A number of candidates omitted the branching methyl group in the amide formed with ethanoic acid and confused aldehydes with ketone and only a small minority referred to the carbonyl group. Most candidates identified only hydrogen bonds in compound C and did not refer to the dipole-dipole forces or van der Waals’ forces also present or explicitly compare the relative strength of the different intermolecular forces in the two molecules. Some incorrectly referred to covalent bonding in their explanation. The equation for the complete combustion of compound C was generally well known. The term stereoisomer was well understood but many candidates did not refer to the restricted rotation around a double bond. Most candidates were able draw the structures of cis and trans but-2-ene.
Most candidates were able to give the full structural formula but marks were lost by some as they gave the condensed formula rather than the full structural formula as demanded by the question. Most were able to apply IUPAC rules and name A but some omitted the “di” from dibromobutane. The colour change observed when but-2-ene reacts with bromine was well known, but knowledge of the economic importance of the polymerisation of alkenes was limited with many candidates restricting their answers to identifying specific plastics such a polythene. Many responses included incorrect references to nylon and margarine. Most candidates were able to identify the repeating unit of poly(but-2-ene). The explanation of the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism was more successful than in previous sessions although a common error was a curly arrow originating from the hydrogen atom in the hydroxide ion rather than the oxygen. Most candidates were able to explain the higher reactivity of the hydroxide ion compared to the water molecule in terms of charge but only a minority referred to the attraction between the nucleophile and low electron density of the carbon atom. The naming of 2-methylbutanenitrile was generally well done although small errors were accepted and the reagents needed for the hydrogenation of 2-methylbutanenitrile were also generally known. A number of candidates omitted the branching methyl group in the amide formed with ethanoic acid and confused aldehydes with ketone and only a small minority referred to the carbonyl group. Most candidates identified only hydrogen bonds in compound C and did not refer to the dipole-dipole forces or van der Waals’ forces also present or explicitly compare the relative strength of the different intermolecular forces in the two molecules. Some incorrectly referred to covalent bonding in their explanation. The equation for the complete combustion of compound C was generally well known. The term stereoisomer was well understood but many candidates did not refer to the restricted rotation around a double bond. Most candidates were able draw the structures of cis and trans but-2-ene.
Most candidates were able to give the full structural formula but marks were lost by some as they gave the condensed formula rather than the full structural formula as demanded by the question. Most were able to apply IUPAC rules and name A but some omitted the “di” from dibromobutane. The colour change observed when but-2-ene reacts with bromine was well known, but knowledge of the economic importance of the polymerisation of alkenes was limited with many candidates restricting their answers to identifying specific plastics such a polythene. Many responses included incorrect references to nylon and margarine. Most candidates were able to identify the repeating unit of poly(but-2-ene). The explanation of the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism was more successful than in previous sessions although a common error was a curly arrow originating from the hydrogen atom in the hydroxide ion rather than the oxygen. Most candidates were able to explain the higher reactivity of the hydroxide ion compared to the water molecule in terms of charge but only a minority referred to the attraction between the nucleophile and low electron density of the carbon atom. The naming of 2-methylbutanenitrile was generally well done although small errors were accepted and the reagents needed for the hydrogenation of 2-methylbutanenitrile were also generally known. A number of candidates omitted the branching methyl group in the amide formed with ethanoic acid and confused aldehydes with ketone and only a small minority referred to the carbonyl group. Most candidates identified only hydrogen bonds in compound C and did not refer to the dipole-dipole forces or van der Waals’ forces also present or explicitly compare the relative strength of the different intermolecular forces in the two molecules. Some incorrectly referred to covalent bonding in their explanation. The equation for the complete combustion of compound C was generally well known. The term stereoisomer was well understood but many candidates did not refer to the restricted rotation around a double bond. Most candidates were able draw the structures of cis and trans but-2-ene.
Most candidates were able to give the full structural formula but marks were lost by some as they gave the condensed formula rather than the full structural formula as demanded by the question. Most were able to apply IUPAC rules and name A but some omitted the “di” from dibromobutane. The colour change observed when but-2-ene reacts with bromine was well known, but knowledge of the economic importance of the polymerisation of alkenes was limited with many candidates restricting their answers to identifying specific plastics such a polythene. Many responses included incorrect references to nylon and margarine. Most candidates were able to identify the repeating unit of poly(but-2-ene). The explanation of the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism was more successful than in previous sessions although a common error was a curly arrow originating from the hydrogen atom in the hydroxide ion rather than the oxygen. Most candidates were able to explain the higher reactivity of the hydroxide ion compared to the water molecule in terms of charge but only a minority referred to the attraction between the nucleophile and low electron density of the carbon atom. The naming of 2-methylbutanenitrile was generally well done although small errors were accepted and the reagents needed for the hydrogenation of 2-methylbutanenitrile were also generally known. A number of candidates omitted the branching methyl group in the amide formed with ethanoic acid and confused aldehydes with ketone and only a small minority referred to the carbonyl group. Most candidates identified only hydrogen bonds in compound C and did not refer to the dipole-dipole forces or van der Waals’ forces also present or explicitly compare the relative strength of the different intermolecular forces in the two molecules. Some incorrectly referred to covalent bonding in their explanation. The equation for the complete combustion of compound C was generally well known. The term stereoisomer was well understood but many candidates did not refer to the restricted rotation around a double bond. Most candidates were able draw the structures of cis and trans but-2-ene.
Most candidates were able to give the full structural formula but marks were lost by some as they gave the condensed formula rather than the full structural formula as demanded by the question. Most were able to apply IUPAC rules and name A but some omitted the “di” from dibromobutane. The colour change observed when but-2-ene reacts with bromine was well known, but knowledge of the economic importance of the polymerisation of alkenes was limited with many candidates restricting their answers to identifying specific plastics such a polythene. Many responses included incorrect references to nylon and margarine. Most candidates were able to identify the repeating unit of poly(but-2-ene). The explanation of the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism was more successful than in previous sessions although a common error was a curly arrow originating from the hydrogen atom in the hydroxide ion rather than the oxygen. Most candidates were able to explain the higher reactivity of the hydroxide ion compared to the water molecule in terms of charge but only a minority referred to the attraction between the nucleophile and low electron density of the carbon atom. The naming of 2-methylbutanenitrile was generally well done although small errors were accepted and the reagents needed for the hydrogenation of 2-methylbutanenitrile were also generally known. A number of candidates omitted the branching methyl group in the amide formed with ethanoic acid and confused aldehydes with ketone and only a small minority referred to the carbonyl group. Most candidates identified only hydrogen bonds in compound C and did not refer to the dipole-dipole forces or van der Waals’ forces also present or explicitly compare the relative strength of the different intermolecular forces in the two molecules. Some incorrectly referred to covalent bonding in their explanation. The equation for the complete combustion of compound C was generally well known. The term stereoisomer was well understood but many candidates did not refer to the restricted rotation around a double bond. Most candidates were able draw the structures of cis and trans but-2-ene.
Most candidates were able to give the full structural formula but marks were lost by some as they gave the condensed formula rather than the full structural formula as demanded by the question. Most were able to apply IUPAC rules and name A but some omitted the “di” from dibromobutane. The colour change observed when but-2-ene reacts with bromine was well known, but knowledge of the economic importance of the polymerisation of alkenes was limited with many candidates restricting their answers to identifying specific plastics such a polythene. Many responses included incorrect references to nylon and margarine. Most candidates were able to identify the repeating unit of poly(but-2-ene). The explanation of the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism was more successful than in previous sessions although a common error was a curly arrow originating from the hydrogen atom in the hydroxide ion rather than the oxygen. Most candidates were able to explain the higher reactivity of the hydroxide ion compared to the water molecule in terms of charge but only a minority referred to the attraction between the nucleophile and low electron density of the carbon atom. The naming of 2-methylbutanenitrile was generally well done although small errors were accepted and the reagents needed for the hydrogenation of 2-methylbutanenitrile were also generally known. A number of candidates omitted the branching methyl group in the amide formed with ethanoic acid and confused aldehydes with ketone and only a small minority referred to the carbonyl group. Most candidates identified only hydrogen bonds in compound C and did not refer to the dipole-dipole forces or van der Waals’ forces also present or explicitly compare the relative strength of the different intermolecular forces in the two molecules. Some incorrectly referred to covalent bonding in their explanation. The equation for the complete combustion of compound C was generally well known. The term stereoisomer was well understood but many candidates did not refer to the restricted rotation around a double bond. Most candidates were able draw the structures of cis and trans but-2-ene.
Most candidates were able to give the full structural formula but marks were lost by some as they gave the condensed formula rather than the full structural formula as demanded by the question. Most were able to apply IUPAC rules and name A but some omitted the “di” from dibromobutane. The colour change observed when but-2-ene reacts with bromine was well known, but knowledge of the economic importance of the polymerisation of alkenes was limited with many candidates restricting their answers to identifying specific plastics such a polythene. Many responses included incorrect references to nylon and margarine. Most candidates were able to identify the repeating unit of poly(but-2-ene). The explanation of the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism was more successful than in previous sessions although a common error was a curly arrow originating from the hydrogen atom in the hydroxide ion rather than the oxygen. Most candidates were able to explain the higher reactivity of the hydroxide ion compared to the water molecule in terms of charge but only a minority referred to the attraction between the nucleophile and low electron density of the carbon atom. The naming of 2-methylbutanenitrile was generally well done although small errors were accepted and the reagents needed for the hydrogenation of 2-methylbutanenitrile were also generally known. A number of candidates omitted the branching methyl group in the amide formed with ethanoic acid and confused aldehydes with ketone and only a small minority referred to the carbonyl group. Most candidates identified only hydrogen bonds in compound C and did not refer to the dipole-dipole forces or van der Waals’ forces also present or explicitly compare the relative strength of the different intermolecular forces in the two molecules. Some incorrectly referred to covalent bonding in their explanation. The equation for the complete combustion of compound C was generally well known. The term stereoisomer was well understood but many candidates did not refer to the restricted rotation around a double bond. Most candidates were able draw the structures of cis and trans but-2-ene.
Most candidates were able to give the full structural formula but marks were lost by some as they gave the condensed formula rather than the full structural formula as demanded by the question. Most were able to apply IUPAC rules and name A but some omitted the “di” from dibromobutane. The colour change observed when but-2-ene reacts with bromine was well known, but knowledge of the economic importance of the polymerisation of alkenes was limited with many candidates restricting their answers to identifying specific plastics such a polythene. Many responses included incorrect references to nylon and margarine. Most candidates were able to identify the repeating unit of poly(but-2-ene). The explanation of the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism was more successful than in previous sessions although a common error was a curly arrow originating from the hydrogen atom in the hydroxide ion rather than the oxygen. Most candidates were able to explain the higher reactivity of the hydroxide ion compared to the water molecule in terms of charge but only a minority referred to the attraction between the nucleophile and low electron density of the carbon atom. The naming of 2-methylbutanenitrile was generally well done although small errors were accepted and the reagents needed for the hydrogenation of 2-methylbutanenitrile were also generally known. A number of candidates omitted the branching methyl group in the amide formed with ethanoic acid and confused aldehydes with ketone and only a small minority referred to the carbonyl group. Most candidates identified only hydrogen bonds in compound C and did not refer to the dipole-dipole forces or van der Waals’ forces also present or explicitly compare the relative strength of the different intermolecular forces in the two molecules. Some incorrectly referred to covalent bonding in their explanation. The equation for the complete combustion of compound C was generally well known. The term stereoisomer was well understood but many candidates did not refer to the restricted rotation around a double bond. Most candidates were able draw the structures of cis and trans but-2-ene.
Most candidates were able to give the full structural formula but marks were lost by some as they gave the condensed formula rather than the full structural formula as demanded by the question. Most were able to apply IUPAC rules and name A but some omitted the “di” from dibromobutane. The colour change observed when but-2-ene reacts with bromine was well known, but knowledge of the economic importance of the polymerisation of alkenes was limited with many candidates restricting their answers to identifying specific plastics such a polythene. Many responses included incorrect references to nylon and margarine. Most candidates were able to identify the repeating unit of poly(but-2-ene). The explanation of the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism was more successful than in previous sessions although a common error was a curly arrow originating from the hydrogen atom in the hydroxide ion rather than the oxygen. Most candidates were able to explain the higher reactivity of the hydroxide ion compared to the water molecule in terms of charge but only a minority referred to the attraction between the nucleophile and low electron density of the carbon atom. The naming of 2-methylbutanenitrile was generally well done although small errors were accepted and the reagents needed for the hydrogenation of 2-methylbutanenitrile were also generally known. A number of candidates omitted the branching methyl group in the amide formed with ethanoic acid and confused aldehydes with ketone and only a small minority referred to the carbonyl group. Most candidates identified only hydrogen bonds in compound C and did not refer to the dipole-dipole forces or van der Waals’ forces also present or explicitly compare the relative strength of the different intermolecular forces in the two molecules. Some incorrectly referred to covalent bonding in their explanation. The equation for the complete combustion of compound C was generally well known. The term stereoisomer was well understood but many candidates did not refer to the restricted rotation around a double bond. Most candidates were able draw the structures of cis and trans but-2-ene.
In an experiment conducted at 25.0 °C, the initial concentration of propanoic acid and methanol were \({\text{1.6 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) and \({\text{2.0 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) respectively. Once equilibrium was established, a sample of the mixture was removed and analysed. It was found to contain \({\text{0.80 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) of compound X.
Two compounds, A and D, each have the formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Cl}}\).
Compound A is reacted with dilute aqueous sodium hydroxide to produce compound B with a formula of \({{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}{\text{O}}\). Compound B is then oxidized with acidified potassium
manganate(VII) to produce compound C with a formula of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{\text{O}}\). Compound C resists further oxidation by acidified potassium manganate(VII).
Compound D is reacted with dilute aqueous sodium hydroxide to produce compound E with a formula of \({{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}{\text{O}}\). Compound E does not react with acidified potassium manganate(VII).
Deduce the structural formulas for compounds A, B, C, D and E.
A:
B:
C:
D:
E:
Deduce an equation for the reaction between propanoic acid and methanol. Identify the catalyst and state the name of the organic compound, X, formed.
Calculate the concentrations of the other three species present at equilibrium.
State the equilibrium constant expression, \({K_{\text{c}}}\), and calculate the equilibrium constant for this reaction at 25.0 °C.
2-chloro-3-methylbutane reacts with sodium hydroxide via an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism. Explain the mechanism by using curly arrows to represent the movement of electron pairs.
Explain why the hydroxide ion is a better nucleophile than water.
1-chlorobutane can be converted to a pentylamine via a two stage process. Deduce equations for each step of this conversion including any catalyst required and name the organic product produced at each stage.
Markscheme
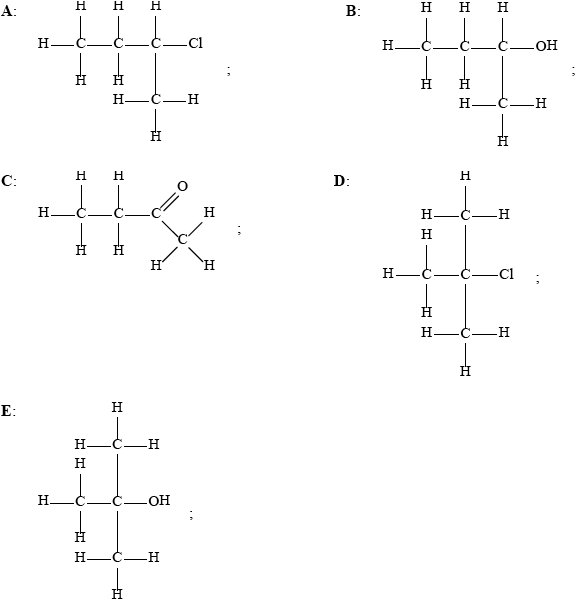
Accept condensed formulas.
Award [1 max] if A and D are other way round (and nothing else correct).
Award [2 max] if A and D are other way round but one substitution product B or E is correct based on initial choice of A and D.
Award [3 max] if A and D are other way round but both substitution products B and E are correct based on initial choice of A and D.
M2 (for B) and M5 (for E) may also be scored for substitution product if primary chloroalkane used.
Penalize missing hydrogens once only in Q.7.
\[{\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{COOH}} + {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}} \rightleftharpoons {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOC}}{{\text{H}}_{\text{3}}} + {{\text{H}}_{\text{2}}}{\text{O}}\]
[1] for reactants and [1] for products.
(concentrated) sulfuric acid/\({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\);
Do not accept just \({H^ + }\) or acid.
methyl propanoate;
[CH3CH2COOH]:
\((1.6 - 0.80 = ){\text{ }}0.8{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
[CH3OH]:
\((2.0 - 0.80 = ){\text{ }}1.2{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
[H2O]:
\({\text{0.80 (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\(({K_{\text{c}}} = )\frac{{{\text{[C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOC}}{{\text{H}}_{\text{3}}}{\text{][}}{{\text{H}}_{\text{2}}}{\text{O]}}}}{{{\text{[C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH][C}}{{\text{H}}_{\text{3}}}{\text{OH]}}}}\);
\(\left( {{K_{\text{c}}} = \frac{{[{{(0.80)}^2}]}}{{\left[ {(1.2 \times 0.8)} \right]}} = } \right){\text{ }}0.7\);
Allow 0.67.
Award [1 max] for 0.83.
curly arrow going from lone pair/negative charge on O in \({\text{H}}{{\text{O}}^ - }\) to C;
Do not allow curly arrow originating on H in \(H{O^ - }\).
curly arrow showing Cl leaving;
Accept curly arrow either going from bond between C and Cl to Cl in 2-chloro-3-methylbutane or in the transition state.
representation of transition state showing negative charge, square brackets and partial bonds;
Do not penalize if HO and Cl are not at 180° to each other.
Do not award M3 if OH ---- C bond is represented.
formation of organic product 3-methylbutan-2-ol and \({\text{C}}{{\text{l}}^ - }\);
\({\text{O}}{{\text{H}}^ - }\) has a negative charge/higher electron density;
greater attraction to the carbon atom (with the partial positive charge) / OWTTE;
Do not allow just greater attraction.
\({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Cl}} + {\text{KCN}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CN}} + {\text{KCl}}\);
Accept \(C{N^ - }\) for KCN and \(C{l^ - }\) for KCl.
pentanenitrile;
Allow 1-cyanobutane.
\({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{CN}} + {\text{2}}{{\text{H}}_2} \to {\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{N}}{{\text{H}}_2}\);
pentan-1-amine / 1-aminopentane / 1-pentylamine / 1-pentanamine;
Catalyst: nickel/Ni / palladium/Pd / platinum/Pt;
Penalise missing hydrogen once only in Q.7.
Examiners report
This was the least popular question in Section B. Most candidates either scored all five marks in (a) or just one.
(b) was usually well done, though it was disappointing that more candidates did not use the equilibrium sign.
In (c), a significant number of candidates omitted water from the equilibrium calculations.
In (c), a significant number of candidates omitted water from the equilibrium calculations.
The organic reaction mechanism in (d) (i) was very poorly presented. Many even tried drawing curly arrows from NaOH as an attacking species. The majority could identify the product of the reaction but a mechanism was far beyond them. Transition states were poor or missing completely.
In (ii) although many knew that \({\text{O}}{{\text{H}}^ - }\) has a negative charge, few linked this to the greater attraction to the carbon atom.
In (iii) very few candidates did well here and the name of pentan-1-amine was rarely given. Other mistakes included incorrect catalysts. Further common mistakes included some candidates not including all the hydrogens in the structural formulas. In general for this part there was very poor knowledge of organic synthesis amongst candidates. Very few had a good “stab” at this question. The fact that pentylamine was mentioned in the question initially meant that very few candidates accessed the last mark for the name of the product.
There are several structural isomers with the molecular formula \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{11}}}}{\text{Br}}\).
All the isomers react when warmed with a dilute aqueous solution of sodium hydroxide according to the equation below.
\[{{\text{C}}_5}{{\text{H}}_{11}}{\text{Br}} + {\text{NaOH}} \to {{\text{C}}_5}{{\text{H}}_{11}}{\text{OH}} + {\text{NaBr}}\]
Deduce the name of one of the isomers which can exist as enantiomers and draw three-dimensional representations of its two enantiomers.
The reaction with 1-bromopentane proceeds by an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism. Describe this mechanism using structural formulas and curly arrows to represent the movement of electron pairs.
The reaction with 2-bromo-2-methylbutane proceeds by an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. Describe this mechanism using structural formulas and curly arrows to represent the movement of electron pairs.
Explain why 1-bromopentane reacts by an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism whereas 2-bromo-2-methylbutane reacts by an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism.
Explain whether the boiling point of 1-bromopentane will be higher, lower or the same as that of 2-bromo-2-methylbutane.
The product \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{11}}}}{\text{OH}}\) formed from the reaction with 1-bromopentane is warmed with ethanoic acid in the presence of a few drops of concentrated sulfuric acid. State the name of the type of reaction taking place and the structural formula of the organic product.
Markscheme
2-bromopentane 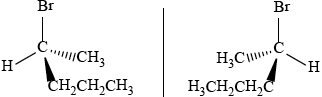
OR
1-bromo-2-methylbutane 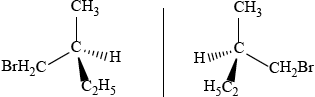
OR
2-bromo-3-methylbutane 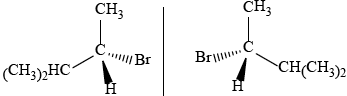
correct isomer 3D structure;
correct name;
correct enantiomer 3D structure;
If compound incorrectly named award [2 max] for two correct 3D enantiomers,
and [1 max] for a correct structure of an enantiomer not shown in 3D.
If non-optically active isomers given (e.g. 2-bromo-2-methyl-butane) award [1 max]
if name and 3D structure are correct.
Accept condensed form for alkyl chain throughout.
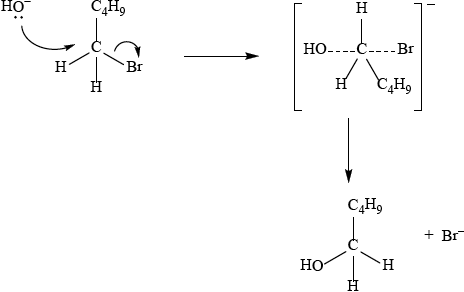
curly arrow going from lone pair/negative charge on O in \({\text{H}}{{\text{O}}^ - }\) to C bonded to Br;
Do not allow curly arrow originating on H in \(H{O^ - }\) (e.g. originating on negative charge on H i.e. lone pair/negative charge must be on O).
curly arrow from C–Br bond to form \({\text{B}}{{\text{r}}^ - }\) (this can also be shown in transition state);
transition state showing overall negative charge;
Accept condensed formulas as long as curly arrows can still be shown e.g.
Accept 
If wrong formula used for halogenoalkane, e.g. 1-bromobutane award [2 max].
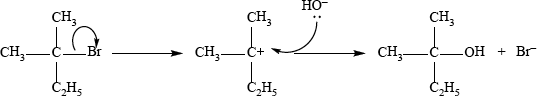
curly arrow from C–Br bond to form \({\text{B}}{{\text{r}}^ - }\);
correct structure of tertiary carbocation;
curly arrow going from lone pair/negative charge on O in \({\text{H}}{{\text{O}}^ - }\) to \({{\text{C}}^ + }\);
If non-bonding pair not shown then arrow must originate from negative sign on O or the minus sign.
Only penalize arrow from H once in (b).
If wrong formula is used for 2-bromo-2-methylbutane award [2 max].
the C bonded to the Br in 1-bromopentane is also bonded to two H atoms so can accommodate five groups around it in the transition state / OWTTE;
the C bonded to the Br in 2-bromo-2-methylbutane has three other (bulky) groups bonded to it so cannot accommodate five groups around it in the transition state / OWTTE;
2-bromo-2-methylbutane forms a tertiary carbocation which is stabilized by the positive inductive effect of the three alkyl groups / OWTTE;
1-bromopentane would form a primary carbocation (if it went by \({{\text{S}}_{\text{N}}}{\text{2}}\)) which is much less stable as there is only one alkyl group exerting a positive inductive effect / OWTTE;
the boiling point of 1-bromopentane is higher than the boiling point of 2-bromo-2-methylbutane;
2-bromo-2-methylbutane is more spherical in shape / less surface area in contact between molecules of 2-bromo-2-methylbutane than between molecules of 1-bromopentane / OWTTE;
hence weaker intermolecular forces of attraction/van der Waals’ forces of attraction between molecules of 2-bromo-2-methylbutane / OWTTE;
esterification / condensation;
\({\text{C}}{{\text{H}}_3}{\text{–CO–O–(C}}{{\text{H}}_2}{{\text{)}}_4}{\text{C}}{{\text{H}}_3}/{\text{C}}{{\text{H}}_3}{\text{COO(C}}{{\text{H}}_2}{{\text{)}}_4}{\text{C}}{{\text{H}}_3}/\)
\({\text{C}}{{\text{H}}_3}{\text{COOC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}/\)
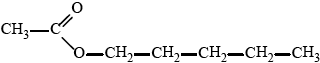 ;
;
Accept CH3–CO–O–C5H11
Examiners report
Although the least popular question, candidates were generally well prepared particularly in drawing enantiomers and describing the mechanisms for the two nucleophilic substitution reactions.
The representation of the \({{\text{S}}_{\text{N}}}{\text{1}}\) and \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanisms using curly arrows has significantly improved from previous sessions but mistakes are still being made.
Common errors in the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism include the curly arrow originating from the H in the hydroxide ion instead of the lone pair on the oxygen and the omission of the negative charge or square brackets from the transition state.
It was also disappointing to see H–C bonds in the transition state and HO–C–Br angles of less than 180°. If a candidate fully understood that the attack must be on the opposite side from the leaving group than this type of mistake would not appear. Explanations of why primary halogenoalkanes undergo \({{\text{S}}_{\text{N}}}{\text{2}}\) reactions and why primary structures favour \({{\text{S}}_{\text{N}}}{\text{1}}\) reactions in terms of steric hindrance and carbocation stability were often incomplete with few candidates gaining full marks. Students should note that when asked to compare two molecules, their answers should refer explicitly to both; i.e. they had to mention that a tertiary compound halogenoalkane did have steric hindrance and a primary compound did not have steric hindrance. Some candidates also struggled to gave a full explanation of the higher boiling point of 1-bromopentane in terms of the greater surface contact between neighbouring molecules. Most candidates were familiar with the esterification reaction and able to give the structural formula of pentyl ethanoate. The prediction of the organic products of the elimination reaction proved to be beyond many, as candidates struggled to apply their knowledge in an unfamiliar context. Similarly, many were unable to give the equation for the condensation polymerisation reaction between benzene-1,4-dicarboxylc acid and pentane-1,5-diol. A significant number of students misread the question and attempted to describe a reaction between the acid and 1,5-dibromopentane instead.
It was also disappointing to see H–C bonds in the transition state and HO–C–Br angles of less than 180°. If a candidate fully understood that the attack must be on the opposite side from the leaving group than this type of mistake would not appear. Explanations of why primary halogenoalkanes undergo \({{\text{S}}_{\text{N}}}{\text{2}}\) reactions and why primary structures favour \({{\text{S}}_{\text{N}}}{\text{1}}\) reactions in terms of steric hindrance and carbocation stability were often incomplete with few candidates gaining full marks. Students should note that when asked to compare two molecules, their answers should refer explicitly to both; i.e. they had to mention that a tertiary compound halogenoalkane did have steric hindrance and a primary compound did not have steric hindrance. Some candidates also struggled to gave a full explanation of the higher boiling point of 1-bromopentane in terms of the greater surface contact between neighbouring molecules. Most candidates were familiar with the esterification reaction and able to give the structural formula of pentyl ethanoate. The prediction of the organic products of the elimination reaction proved to be beyond many, as candidates struggled to apply their knowledge in an unfamiliar context. Similarly, many were unable to give the equation for the condensation polymerisation reaction between benzene-1,4-dicarboxylc acid and pentane-1,5-diol. A significant number of students misread the question and attempted to describe a reaction between the acid and 1,5-dibromopentane instead.
It was also disappointing to see H–C bonds in the transition state and HO–C–Br angles of less than 180°. If a candidate fully understood that the attack must be on the opposite side from the leaving group than this type of mistake would not appear. Explanations of why primary halogenoalkanes undergo \({{\text{S}}_{\text{N}}}{\text{2}}\) reactions and why primary structures favour \({{\text{S}}_{\text{N}}}{\text{1}}\) reactions in terms of steric hindrance and carbocation stability were often incomplete with few candidates gaining full marks. Students should note that when asked to compare two molecules, their answers should refer explicitly to both; i.e. they had to mention that a tertiary compound halogenoalkane did have steric hindrance and a primary compound did not have steric hindrance. Some candidates also struggled to gave a full explanation of the higher boiling point of 1-bromopentane in terms of the greater surface contact between neighbouring molecules. Most candidates were familiar with the esterification reaction and able to give the structural formula of pentyl ethanoate. The prediction of the organic products of the elimination reaction proved to be beyond many, as candidates struggled to apply their knowledge in an unfamiliar context. Similarly, many were unable to give the equation for the condensation polymerisation reaction between benzene-1,4-dicarboxylc acid and pentane-1,5-diol. A significant number of students misread the question and attempted to describe a reaction between the acid and 1,5-dibromopentane instead.
It was also disappointing to see H–C bonds in the transition state and HO–C–Br angles of less than 180°. If a candidate fully understood that the attack must be on the opposite side from the leaving group than this type of mistake would not appear. Explanations of why primary halogenoalkanes undergo \({{\text{S}}_{\text{N}}}{\text{2}}\) reactions and why primary structures favour \({{\text{S}}_{\text{N}}}{\text{1}}\) reactions in terms of steric hindrance and carbocation stability were often incomplete with few candidates gaining full marks. Students should note that when asked to compare two molecules, their answers should refer explicitly to both; i.e. they had to mention that a tertiary compound halogenoalkane did have steric hindrance and a primary compound did not have steric hindrance. Some candidates also struggled to gave a full explanation of the higher boiling point of 1-bromopentane in terms of the greater surface contact between neighbouring molecules. Most candidates were familiar with the esterification reaction and able to give the structural formula of pentyl ethanoate. The prediction of the organic products of the elimination reaction proved to be beyond many, as candidates struggled to apply their knowledge in an unfamiliar context. Similarly, many were unable to give the equation for the condensation polymerisation reaction between benzene-1,4-dicarboxylc acid and pentane-1,5-diol. A significant number of students misread the question and attempted to describe a reaction between the acid and 1,5-dibromopentane instead.
Iron rusts in the presence of oxygen and water. Rusting is a redox process involving several steps that produces hydrated iron(III) oxide, \({\text{F}}{{\text{e}}_{\text{2}}}{{\text{O}}_{\text{3}}} \bullet {\text{n}}{{\text{H}}_{\text{2}}}{\text{O}}\), as the final product.
The half-equations involved for the first step of rusting are given below.
Half-equation 1: \({\text{Fe(s)}} \to {\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - }\)
Half-equation 2: \({{\text{O}}_{\text{2}}}{\text{(aq)}} + {\text{4}}{{\text{e}}^ - } + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} \to {\text{4O}}{{\text{H}}^ - }{\text{(aq)}}\)
(i) Identify whether half-equation 1 represents oxidation or reduction, giving a reason for your answer.
(ii) Identify the oxidation number of each atom in the three species in half-equation 2.
(iii) Deduce the overall redox equation for the first step of rusting by combining half-equations 1 and 2.
(iv) Identify the reducing agent in the redox equation in part (iii).
The oxygen in half-equation 2 is atmospheric oxygen that is found dissolved in water in very small concentrations. Explain, in terms of intermolecular forces, why oxygen is not very soluble in water.
State the relationship between the electron arrangement of an element and its group and period in the periodic table.
Transition metals and their compounds often catalyse reactions. The catalyzed decomposition of hydrogen peroxide by CuO is an example. State two other examples of catalyzed reactions giving the transition metal or its compound acting as catalyst.
(i) State a chemical equation for the partial dissociation of water into ions, including state symbols.
(ii) The dissociation of water into ions is reversible. State the expression for the ionic product constant of water.
(iii) The ionic product constant of water was measured at three different temperatures.
Deduce whether the ionization of water is exothermic or endothermic, giving your reason.
(iv) Use the data in part (iii) to determine the pH of water at 373 K, correct to two decimal places.
(i) An aqueous solution of sodium chloride is electrolysed using inert electrodes. Explain which product is obtained at the positive electrode (anode) if the concentration of sodium chloride is high.
(ii) State the half-equations occurring at the electrodes during the electrolysis of the concentrated aqueous solution of sodium chloride.
Negative electrode (cathode):
Positive electrode (anode):
Describe how electrolysis can be used to electroplate a bracelet with a layer of silver metal. Include the choice of electrodes and electrolyte needed in your description.
Markscheme
(i) oxidation and (iron/Fe) loses electrons/increases in oxidation number/state;
(ii)  ;
;
Award [2] for five correct.
Award [1] for four correct.
Accept use of oxidation states (0, +1, –2, –2, +1) for oxidation numbers.
Penalize once for incorrect notation (eg, 2, 2–).
(iii) \({{\text{O}}_2}{\text{(aq)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}} + {\text{2Fe(s)}} \to {\text{2F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{4O}}{{\text{H}}^ - }{\text{(aq)}}\);
Ignore state symbols.
(iv) Fe/iron;
needs to break strong hydrogen bonds/H–bonds between water molecules (to dissolve) / oxygen cannot form hydrogen bonds/H–bonds with water;
oxygen can only form (weak) van der Waals’/vdW/LDF/London/dispersion forces with water;
groups indicate the number of electrons in the highest energy level/outer/valence shell;
periods indicate the number of (occupied) energy levels/shells (in the atom);
\({{\text{V}}_{\text{2}}}{{\text{O}}_{\text{5}}}\) catalyses oxidation of \({\text{S}}{{\text{O}}_{\text{2}}}\) / \({{\text{V}}_{\text{2}}}{{\text{O}}_{\text{5}}}\) is a catalyst in the Contact Process;
Fe catalyses the reaction between \({{\text{N}}_{\text{2}}}\) and \({{\text{H}}_{\text{2}}}\) / Fe is a catalyst in the Haber Process;
Ni/Pd/Pt catalyses hydrogenation / manufacture of margarine / addition of hydrogen to C=C / conversion of alkenes to alkanes;
Pd/Pt is a catalyst in catalytic converters / Pd/Pt catalyzes reaction of \({\text{N}}{{\text{O}}_{\text{2}}}\) and CO/\({\text{N}}{{\text{O}}_{\text{2}}}\) and (unburnt) fuel/exhaust gases;
Accept other correct examples.
Accept formulas or names of substances.
(i) \({{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {{\text{H}}^ + }{\text{(aq)}} + {\text{O}}{{\text{H}}^ - }{\text{(aq)}}/{\text{2}}{{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {{\text{H}}_3}{{\text{O}}^ + }{\text{(aq)}} + {\text{O}}{{\text{H}}^ - }{\text{(aq)}}\);
\( \rightleftharpoons \) and state symbols are necessary for the mark.
(ii) \({K_w} = {\text{[}}{{\text{H}}^ + }{\text{][O}}{{\text{H}}^ - }{\text{]}}/{K_w} = {\text{[}}{{\text{H}}_3}{{\text{O}}^ + }{\text{][O}}{{\text{H}}^ - }{\text{]}}\);
(iii) at higher temperatures ionization increases / at higher temperatures equilibrium shifts to right;
ionization is endothermic;
Do not allow ECF for M2.
(iv) \({\text{5.13}} \times {\text{1}}{{\text{0}}^{ - 13}} = {{\text{[}}{{\text{H}}_3}{{\text{O}}^ + }{\text{]}}^2}/{{\text{[}}{{\text{H}}^ + }{\text{]}}^2}/{\text{[}}{{\text{H}}_3}{{\text{O}}^ + }{\text{]}}/{\text{[}}{{\text{H}}^ + }{\text{]}} = {\text{7.16}} \times {\text{1}}{{\text{0}}^{ - 7}}{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\({\text{pH}} = 6.14/6.15\);
Award [2] for correct final answer.
(i) chlorine/\({\text{C}}{{\text{l}}_{\text{2}}}\) (is produced at the positive electrode/anode);
according to electrochemical series/ \(E^\circ \) values/ease of oxidation \({\text{O}}{{\text{H}}^ - }\)/\({{\text{H}}_{\text{2}}}{\text{O}}\) reacts/oxygen is released / OWTTE / at low chloride concentration \({\text{O}}{{\text{H}}^ - }\)/\({{\text{H}}_{\text{2}}}{\text{O}}\) reacts/oxygen is released;
high concentration makes \({\text{C}}{{\text{l}}^ - }\) oxidize/react in preference to \({\text{O}}{{\text{H}}^ - }\)/\({{\text{H}}_{\text{2}}}{\text{O}}\) / OWTTE;
(ii) Negative electrode (cathode):
\({\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \to {{\text{H}}_2}{\text{(g)}}/{{\text{H}}^ + }{\text{(aq)}} + {{\text{e}}^ - } \to \frac{1}{2}{{\text{H}}_2}{\text{(g)}}/{\text{2}}{{\text{H}}_2}{\text{O(l)}} + {\text{2}}{{\text{e}}^ - } \to {{\text{H}}_2}{\text{(g)}} + {\text{2O}}{{\text{H}}^ - }{\text{(aq)}}\);
Positive electrode (anode):
\({\text{2C}}{{\text{l}}^ - }{\text{(aq)}} \to {\text{C}}{{\text{l}}_2}{\text{(g)}} + {\text{2}}{{\text{e}}^ - }/{\text{C}}{{\text{l}}^ - }{\text{(aq)}} \to \frac{1}{2}{\text{C}}{{\text{l}}_2}{\text{(g)}} + {{\text{e}}^ - }/{\text{2C}}{{\text{l}}^ - }{\text{(aq)}} - {\text{2}}{{\text{e}}^ - } \to {\text{C}}{{\text{l}}_2}{\text{(g)}}/\)
\({\text{C}}{{\text{l}}^ - }{\text{(aq)}} - {{\text{e}}^ - } \to \frac{1}{2}{\text{C}}{{\text{l}}_2}{\text{(g)}}\);
Ignore state symbols.
Accept e instead of e–.
Award [1] if half-equations are correct but placed at the wrong electrodes.
bracelet/object to be electroplated is the cathode/negative electrode;
silver anode/positive electrode;
Accept Pt anode.
Electrolyte: liquid \({\text{Na[Ag(C}}{{\text{N}}_{\text{2}}}{\text{)]}}\)/sodium dicyanoargentate/\({{\text{[Ag(CN}}{{\text{)}}_{\text{2}}}{\text{]}}^ - }\)/ solution of an appropriate silver salt;
Accept AgNO3/silver nitrate.
All marks can be scored with a labelled diagram.
Examiners report
(i) Very well answered.
(ii) Most candidates answered correctly. The most common mistakes were doubling the oxidation number of H in \({{\text{H}}_{\text{2}}}{\text{O}}\), and entering a wrong oxidation number for elemental oxygen.
(iii) A well-answered question.
The aqueous solubility of oxygen gas was often poorly explained, with the discussion focussing on the intermolecular forces found in each substance separately and then stating that “like dissolves like”.
Well answered by most candidates.
The majority of candidates were able to give two valid examples of transition metals or their compounds acting as catalysts.
(i) Very well answered.
(ii) Well answered.
(iii) About half of the candidates were able to gain full marks. Some candidates found difficulty in connecting the increase in \({K_{\text{w}}}\) to the position of equilibrium.
(iv) About half of the candidates were able to calculate the pH from the \({K_{\text{w}}}\) value.
(i) Many candidates identified chlorine as the product, but the other two marks were more discriminating. Some candidates clarified that \({\text{C}}{{\text{l}}^ - }\) was oxidized in preference to OH- because of its high concentration, but very few related the situation to the electrochemical series.
(ii) This was poorly answered by many candidates. Common mistakes included releasing sodium at the cathode, reversing electrodes and unbalanced redox half-reactions where the electrons were sometimes on the wrong side of the equation.
Very well answered. Most candidates determined both electrodes correctly. The main difficulty for some candidates was choosing a suitable electrolyte.
Ethanol is a primary alcohol that can be oxidized by acidified potassium dichromate(VI). Distinguish between the reaction conditions needed to produce ethanal and ethanoic acid.
Ethanal:
Ethanoic acid:
Determine the oxidation number of carbon in ethanol and ethanal.
Ethanol:
Ethanal:
Deduce the half-equation for the oxidation of ethanol to ethanal.
Deduce the overall redox equation for the reaction of ethanol to ethanal with acidified potassium dichromate(VI).
Ethanol can be made by reacting aqueous sodium hydroxide with bromoethane.
Explain the mechanism for this reaction, using curly arrows to represent the movement of electron pairs.
Determine the orders of reaction of the reactants and the overall rate expression for the reaction between 2-bromobutane and aqueous sodium hydroxide using the data in the table.
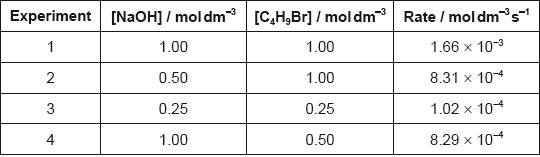
Determine the rate constant, \(k\), with its units, using the data from experiment 3.
Identify the molecularity of the rate-determining step in this reaction.
2-bromobutane exists as optical isomers.
State the essential feature of optical isomers.
2-bromobutane exists as optical isomers.
Outline how a polarimeter can distinguish between these isomers.
Describe the formation of \(\sigma \) and \(\pi \) bonds in an alkene.
The two most abundant isotopes of bromine have the mass numbers 79 and 81.
Calculate the relative abundance of \(^{{\text{79}}}{\text{Br}}\) using table 5 of the data booklet, assuming the abundance of the other isotopes is negligible.
Markscheme
Ethanal: distill off product as it forms;
Accept distillation.
Ethanoic acid: (heat under) reflux / use excess oxidizing agent;
Ethanol: –2/–II;
Ethanal: –1/–I;
Do not accept 2–, 1– but penalize once only.
\({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}} \to {\text{C}}{{\text{H}}_3}{\text{CHO}} + {\text{2}}{{\text{H}}^ + } + {\text{2}}{{\text{e}}^ - }\);
Half-equation required. Do not accept \({C_2}{H_5}OH + 2[O] \to C{H_3}CHO + {H_2}O\).
Accept e for \({e^ - }\).
\({\text{3C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH(aq)}} + {\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 - }{\text{(aq)}} + {\text{8}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{2C}}{{\text{r}}^{{\text{3}} + }}{\text{(aq)}} + {\text{3C}}{{\text{H}}_3}{\text{CHO(l)}} + {\text{7}}{{\text{H}}_2}{\text{O(l)}}\)
correct reactants and products;
correct balancing;
M2 can only be scored if M1 correct.
Ignore state symbols.
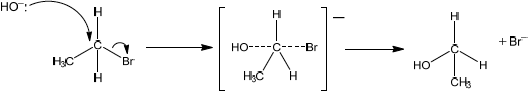
curly arrow going from lone pair/negative charge on O in \({\text{H}}{{\text{O}}^ - }\) to C;
Do not allow curly arrow originating on H in \(H{O^ - }\).
curly arrow showing Br leaving;
Accept curly arrow either going from bond between C and Br to Br in bromoethane or in the transition state.
representation of transition state showing negative charge, square brackets and partial bonds;
Do not penalize if HO and Br are not at 180° to each other.
Do not award M3 if OH----C bond is represented.
formation of organic product \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\) and \({\text{B}}{{\text{r}}^ - }\);
Award [3 max] for correct \({S_N}1\) mechanism.
\({\text{[NaOH] / [O}}{{\text{H}}^ - }{\text{]}}\) is 1/first order and \({\text{[}}{{\text{C}}_4}{{\text{H}}_9}{\text{Br]}}\) is 1/first order;
\({\text{rate }} = k{\text{[O}}{{\text{H}}^ - }{\text{][}}{{\text{C}}_4}{{\text{H}}_9}{\text{Br] / rate}} = k{\text{[NaOH][}}{{\text{C}}_4}{{\text{H}}_9}{\text{Br]}}\);
Square brackets must be used for M2.
\(\left( {\frac{{1.02 \times {{10}^{ - 4}}}}{{0.25 \times 0.25}} = } \right)0.0016/1.6 \times {10^{ - 3}}\);
\({\text{mo}}{{\text{l}}^{ - 1}}\,{\text{d}}{{\text{m}}^{\text{3}}}\,{{\text{s}}^{ - 1}}\);
Accept \({M^{ - 1}} {s^{ - 1}}\).
Ignore order of units.
Must use experiment 3 data.
bimolecular/2;
Accept dimolecular.
chiral/asymmetric carbon / carbon attached to 4 different groups / non-super imposable mirror images;
enantiomers rotate plane of (plane-) polarized light;
in opposite directions (by equal amounts);
Sigma bonds:
result from head-on/end-on overlap of orbitals / OWTTE;
Accept axial overlap of orbitals.
Accept “symmetric orbital” with respect to same plane / OWTTE.
Pi bonds:
result from sideways overlap of orbitals / OWTTE;
Accept “antisymmetric orbitals” with respect to (defining) plane (containing at least one atom) / OWTTE.
\(79.91 = 79x + 81(1 - x)\);
Award M1 for any suitable calculation.
(abundance \(^{{\text{79}}}{\text{Br}} = \)) 54.5%;
Award [2] for correct final answer.
Examiners report
The idea of “reflux” was usually given for the production of ethanoic acid in (a) but ethanal was less clear. We accept that perhaps we should have phrased (a) (ii), “Determine the average oxidation number of carbon in …” In practice, this was one of the best answered parts and caused few difficulties. Few had any idea how to attempt the half-equation in (iii) and the overall equation in (iv). Although the mechanism in (b) has been set on numerous occasions, candidates are still not taking care over the start and finish of the curly arrows and the intermediate is drawn poorly. It must have partial bonds and the sign must be outside the square brackets. Some candidates offered an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. In (c) (ii), the orders were usually successfully deduced but many omitted to give the overall rate expression. In part (ii), quite a number of candidates unaccountably ignored the instruction and used any experiment but No 3. The units were frequently wrong or omitted. The molecularity was answered satisfactorily. In (d), candidates frequently stated that the molecules have mirror images but not that these mirror images are non-superposable. “Chiral” was a popular correct answer. There seemed to be little understanding of a polarimeter with some suggesting that the crystals themselves rotate. In (e) the equations were poor and few were able to identify the reagent. Most descriptions in (f) would have been improved with a careful and clear diagram. Part (g), the relative abundance of \(^{{\text{79}}}{\text{Br}}\) was well done except by those who tried to do it “by inspection”; this usually led to the wrong answer.
The idea of “reflux” was usually given for the production of ethanoic acid in (a) but ethanal was less clear. We accept that perhaps we should have phrased (a) (ii), “Determine the average oxidation number of carbon in …” In practice, this was one of the best answered parts and caused few difficulties. Few had any idea how to attempt the half-equation in (iii) and the overall equation in (iv). Although the mechanism in (b) has been set on numerous occasions, candidates are still not taking care over the start and finish of the curly arrows and the intermediate is drawn poorly. It must have partial bonds and the sign must be outside the square brackets. Some candidates offered an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. In (c) (ii), the orders were usually successfully deduced but many omitted to give the overall rate expression. In part (ii), quite a number of candidates unaccountably ignored the instruction and used any experiment but No 3. The units were frequently wrong or omitted. The molecularity was answered satisfactorily. In (d), candidates frequently stated that the molecules have mirror images but not that these mirror images are non-superposable. “Chiral” was a popular correct answer. There seemed to be little understanding of a polarimeter with some suggesting that the crystals themselves rotate. In (e) the equations were poor and few were able to identify the reagent. Most descriptions in (f) would have been improved with a careful and clear diagram. Part (g), the relative abundance of \(^{{\text{79}}}{\text{Br}}\) was well done except by those who tried to do it “by inspection”; this usually led to the wrong answer.
The idea of “reflux” was usually given for the production of ethanoic acid in (a) but ethanal was less clear. We accept that perhaps we should have phrased (a) (ii), “Determine the average oxidation number of carbon in …” In practice, this was one of the best answered parts and caused few difficulties. Few had any idea how to attempt the half-equation in (iii) and the overall equation in (iv). Although the mechanism in (b) has been set on numerous occasions, candidates are still not taking care over the start and finish of the curly arrows and the intermediate is drawn poorly. It must have partial bonds and the sign must be outside the square brackets. Some candidates offered an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. In (c) (ii), the orders were usually successfully deduced but many omitted to give the overall rate expression. In part (ii), quite a number of candidates unaccountably ignored the instruction and used any experiment but No 3. The units were frequently wrong or omitted. The molecularity was answered satisfactorily. In (d), candidates frequently stated that the molecules have mirror images but not that these mirror images are non-superposable. “Chiral” was a popular correct answer. There seemed to be little understanding of a polarimeter with some suggesting that the crystals themselves rotate. In (e) the equations were poor and few were able to identify the reagent. Most descriptions in (f) would have been improved with a careful and clear diagram. Part (g), the relative abundance of \(^{{\text{79}}}{\text{Br}}\) was well done except by those who tried to do it “by inspection”; this usually led to the wrong answer.
The idea of “reflux” was usually given for the production of ethanoic acid in (a) but ethanal was less clear. We accept that perhaps we should have phrased (a) (ii), “Determine the average oxidation number of carbon in …” In practice, this was one of the best answered parts and caused few difficulties. Few had any idea how to attempt the half-equation in (iii) and the overall equation in (iv). Although the mechanism in (b) has been set on numerous occasions, candidates are still not taking care over the start and finish of the curly arrows and the intermediate is drawn poorly. It must have partial bonds and the sign must be outside the square brackets. Some candidates offered an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. In (c) (ii), the orders were usually successfully deduced but many omitted to give the overall rate expression. In part (ii), quite a number of candidates unaccountably ignored the instruction and used any experiment but No 3. The units were frequently wrong or omitted. The molecularity was answered satisfactorily. In (d), candidates frequently stated that the molecules have mirror images but not that these mirror images are non-superposable. “Chiral” was a popular correct answer. There seemed to be little understanding of a polarimeter with some suggesting that the crystals themselves rotate. In (e) the equations were poor and few were able to identify the reagent. Most descriptions in (f) would have been improved with a careful and clear diagram. Part (g), the relative abundance of \(^{{\text{79}}}{\text{Br}}\) was well done except by those who tried to do it “by inspection”; this usually led to the wrong answer.
The idea of “reflux” was usually given for the production of ethanoic acid in (a) but ethanal was less clear. We accept that perhaps we should have phrased (a) (ii), “Determine the average oxidation number of carbon in …” In practice, this was one of the best answered parts and caused few difficulties. Few had any idea how to attempt the half-equation in (iii) and the overall equation in (iv). Although the mechanism in (b) has been set on numerous occasions, candidates are still not taking care over the start and finish of the curly arrows and the intermediate is drawn poorly. It must have partial bonds and the sign must be outside the square brackets. Some candidates offered an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. In (c) (ii), the orders were usually successfully deduced but many omitted to give the overall rate expression. In part (ii), quite a number of candidates unaccountably ignored the instruction and used any experiment but No 3. The units were frequently wrong or omitted. The molecularity was answered satisfactorily. In (d), candidates frequently stated that the molecules have mirror images but not that these mirror images are non-superposable. “Chiral” was a popular correct answer. There seemed to be little understanding of a polarimeter with some suggesting that the crystals themselves rotate. In (e) the equations were poor and few were able to identify the reagent. Most descriptions in (f) would have been improved with a careful and clear diagram. Part (g), the relative abundance of \(^{{\text{79}}}{\text{Br}}\) was well done except by those who tried to do it “by inspection”; this usually led to the wrong answer.
The idea of “reflux” was usually given for the production of ethanoic acid in (a) but ethanal was less clear. We accept that perhaps we should have phrased (a) (ii), “Determine the average oxidation number of carbon in …” In practice, this was one of the best answered parts and caused few difficulties. Few had any idea how to attempt the half-equation in (iii) and the overall equation in (iv). Although the mechanism in (b) has been set on numerous occasions, candidates are still not taking care over the start and finish of the curly arrows and the intermediate is drawn poorly. It must have partial bonds and the sign must be outside the square brackets. Some candidates offered an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. In (c) (ii), the orders were usually successfully deduced but many omitted to give the overall rate expression. In part (ii), quite a number of candidates unaccountably ignored the instruction and used any experiment but No 3. The units were frequently wrong or omitted. The molecularity was answered satisfactorily. In (d), candidates frequently stated that the molecules have mirror images but not that these mirror images are non-superposable. “Chiral” was a popular correct answer. There seemed to be little understanding of a polarimeter with some suggesting that the crystals themselves rotate. In (e) the equations were poor and few were able to identify the reagent. Most descriptions in (f) would have been improved with a careful and clear diagram. Part (g), the relative abundance of \(^{{\text{79}}}{\text{Br}}\) was well done except by those who tried to do it “by inspection”; this usually led to the wrong answer.
The idea of “reflux” was usually given for the production of ethanoic acid in (a) but ethanal was less clear. We accept that perhaps we should have phrased (a) (ii), “Determine the average oxidation number of carbon in …” In practice, this was one of the best answered parts and caused few difficulties. Few had any idea how to attempt the half-equation in (iii) and the overall equation in (iv). Although the mechanism in (b) has been set on numerous occasions, candidates are still not taking care over the start and finish of the curly arrows and the intermediate is drawn poorly. It must have partial bonds and the sign must be outside the square brackets. Some candidates offered an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. In (c) (ii), the orders were usually successfully deduced but many omitted to give the overall rate expression. In part (ii), quite a number of candidates unaccountably ignored the instruction and used any experiment but No 3. The units were frequently wrong or omitted. The molecularity was answered satisfactorily. In (d), candidates frequently stated that the molecules have mirror images but not that these mirror images are non-superposable. “Chiral” was a popular correct answer. There seemed to be little understanding of a polarimeter with some suggesting that the crystals themselves rotate. In (e) the equations were poor and few were able to identify the reagent. Most descriptions in (f) would have been improved with a careful and clear diagram. Part (g), the relative abundance of \(^{{\text{79}}}{\text{Br}}\) was well done except by those who tried to do it “by inspection”; this usually led to the wrong answer.
The idea of “reflux” was usually given for the production of ethanoic acid in (a) but ethanal was less clear. We accept that perhaps we should have phrased (a) (ii), “Determine the average oxidation number of carbon in …” In practice, this was one of the best answered parts and caused few difficulties. Few had any idea how to attempt the half-equation in (iii) and the overall equation in (iv). Although the mechanism in (b) has been set on numerous occasions, candidates are still not taking care over the start and finish of the curly arrows and the intermediate is drawn poorly. It must have partial bonds and the sign must be outside the square brackets. Some candidates offered an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. In (c) (ii), the orders were usually successfully deduced but many omitted to give the overall rate expression. In part (ii), quite a number of candidates unaccountably ignored the instruction and used any experiment but No 3. The units were frequently wrong or omitted. The molecularity was answered satisfactorily. In (d), candidates frequently stated that the molecules have mirror images but not that these mirror images are non-superposable. “Chiral” was a popular correct answer. There seemed to be little understanding of a polarimeter with some suggesting that the crystals themselves rotate. In (e) the equations were poor and few were able to identify the reagent. Most descriptions in (f) would have been improved with a careful and clear diagram. Part (g), the relative abundance of \(^{{\text{79}}}{\text{Br}}\) was well done except by those who tried to do it “by inspection”; this usually led to the wrong answer.
The idea of “reflux” was usually given for the production of ethanoic acid in (a) but ethanal was less clear. We accept that perhaps we should have phrased (a) (ii), “Determine the average oxidation number of carbon in …” In practice, this was one of the best answered parts and caused few difficulties. Few had any idea how to attempt the half-equation in (iii) and the overall equation in (iv). Although the mechanism in (b) has been set on numerous occasions, candidates are still not taking care over the start and finish of the curly arrows and the intermediate is drawn poorly. It must have partial bonds and the sign must be outside the square brackets. Some candidates offered an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. In (c) (ii), the orders were usually successfully deduced but many omitted to give the overall rate expression. In part (ii), quite a number of candidates unaccountably ignored the instruction and used any experiment but No 3. The units were frequently wrong or omitted. The molecularity was answered satisfactorily. In (d), candidates frequently stated that the molecules have mirror images but not that these mirror images are non-superposable. “Chiral” was a popular correct answer. There seemed to be little understanding of a polarimeter with some suggesting that the crystals themselves rotate. In (e) the equations were poor and few were able to identify the reagent. Most descriptions in (f) would have been improved with a careful and clear diagram. Part (g), the relative abundance of \(^{{\text{79}}}{\text{Br}}\) was well done except by those who tried to do it “by inspection”; this usually led to the wrong answer.
The idea of “reflux” was usually given for the production of ethanoic acid in (a) but ethanal was less clear. We accept that perhaps we should have phrased (a) (ii), “Determine the average oxidation number of carbon in …” In practice, this was one of the best answered parts and caused few difficulties. Few had any idea how to attempt the half-equation in (iii) and the overall equation in (iv). Although the mechanism in (b) has been set on numerous occasions, candidates are still not taking care over the start and finish of the curly arrows and the intermediate is drawn poorly. It must have partial bonds and the sign must be outside the square brackets. Some candidates offered an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. In (c) (ii), the orders were usually successfully deduced but many omitted to give the overall rate expression. In part (ii), quite a number of candidates unaccountably ignored the instruction and used any experiment but No 3. The units were frequently wrong or omitted. The molecularity was answered satisfactorily. In (d), candidates frequently stated that the molecules have mirror images but not that these mirror images are non-superposable. “Chiral” was a popular correct answer. There seemed to be little understanding of a polarimeter with some suggesting that the crystals themselves rotate. In (e) the equations were poor and few were able to identify the reagent. Most descriptions in (f) would have been improved with a careful and clear diagram. Part (g), the relative abundance of \(^{{\text{79}}}{\text{Br}}\) was well done except by those who tried to do it “by inspection”; this usually led to the wrong answer.
The idea of “reflux” was usually given for the production of ethanoic acid in (a) but ethanal was less clear. We accept that perhaps we should have phrased (a) (ii), “Determine the average oxidation number of carbon in …” In practice, this was one of the best answered parts and caused few difficulties. Few had any idea how to attempt the half-equation in (iii) and the overall equation in (iv). Although the mechanism in (b) has been set on numerous occasions, candidates are still not taking care over the start and finish of the curly arrows and the intermediate is drawn poorly. It must have partial bonds and the sign must be outside the square brackets. Some candidates offered an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. In (c) (ii), the orders were usually successfully deduced but many omitted to give the overall rate expression. In part (ii), quite a number of candidates unaccountably ignored the instruction and used any experiment but No 3. The units were frequently wrong or omitted. The molecularity was answered satisfactorily. In (d), candidates frequently stated that the molecules have mirror images but not that these mirror images are non-superposable. “Chiral” was a popular correct answer. There seemed to be little understanding of a polarimeter with some suggesting that the crystals themselves rotate. In (e) the equations were poor and few were able to identify the reagent. Most descriptions in (f) would have been improved with a careful and clear diagram. Part (g), the relative abundance of \(^{{\text{79}}}{\text{Br}}\) was well done except by those who tried to do it “by inspection”; this usually led to the wrong answer.
The idea of “reflux” was usually given for the production of ethanoic acid in (a) but ethanal was less clear. We accept that perhaps we should have phrased (a) (ii), “Determine the average oxidation number of carbon in …” In practice, this was one of the best answered parts and caused few difficulties. Few had any idea how to attempt the half-equation in (iii) and the overall equation in (iv). Although the mechanism in (b) has been set on numerous occasions, candidates are still not taking care over the start and finish of the curly arrows and the intermediate is drawn poorly. It must have partial bonds and the sign must be outside the square brackets. Some candidates offered an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. In (c) (ii), the orders were usually successfully deduced but many omitted to give the overall rate expression. In part (ii), quite a number of candidates unaccountably ignored the instruction and used any experiment but No 3. The units were frequently wrong or omitted. The molecularity was answered satisfactorily. In (d), candidates frequently stated that the molecules have mirror images but not that these mirror images are non-superposable. “Chiral” was a popular correct answer. There seemed to be little understanding of a polarimeter with some suggesting that the crystals themselves rotate. In (e) the equations were poor and few were able to identify the reagent. Most descriptions in (f) would have been improved with a careful and clear diagram. Part (g), the relative abundance of \(^{{\text{79}}}{\text{Br}}\) was well done except by those who tried to do it “by inspection”; this usually led to the wrong answer.
Consider the following sequence of reactions.
\[{\text{RC}}{{\text{H}}_3}\xrightarrow{{reaction 1}}{\text{RC}}{{\text{H}}_2}{\text{Br}}\xrightarrow{{reaction 2}}{\text{RC}}{{\text{H}}_2}{\text{OH}}\]
\({\text{RC}}{{\text{H}}_{\text{3}}}\) is an unknown alkane in which R represents an alkyl group.
All the isomers can by hydrolysed with aqueous sodium hydroxide solution. When the reaction of one of these isomers, X, was investigated the following kinetic data were obtained.
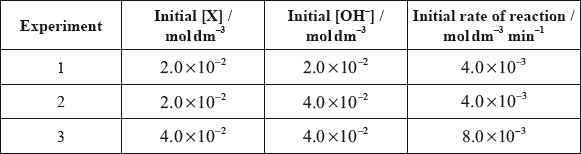
The alkane contains 82.6% by mass of carbon. Determine its empirical formula, showing your working.
A 1.00 g gaseous sample of the alkane has a volume of 385 cm3 at standard temperature and pressure. Deduce its molecular formula.
State the reagent and conditions needed for reaction 1.
Reaction 1 involves a free-radical mechanism. Describe the stepwise mechanism, by giving equations to represent the initiation, propagation and termination steps.
The mechanism in reaction 2 is described as SN2. Explain the mechanism of this reaction using curly arrows to show the movement of electron pairs, and draw the structure of the transition state.
There are four structural isomers with the molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\). One of these structural isomers exists as two optical isomers. Draw diagrams to represent the three-dimensional structures of the two optical isomers.
(i) Deduce the rate expression for the reaction.
(ii) Determine the value of the rate constant for the reaction and state its units.
(iii) State the name of isomer X and explain your choice.
(iv) State equations for the steps that take place in the mechanism of this reaction and state which of the steps is slow and which is fast.
Markscheme
\({n_{\text{C}}} = \frac{{82.6}}{{12.01}} = 6.88\) and \({n_{\text{H}}} = \frac{{17.4}}{{1.01}} = 17.2\);
ratio is 1:2.5;
\({{\text{C}}_2}{{\text{H}}_5}\);
No penalty for using 12 and 1.
\(\left( {M = \frac{{22400}}{{385}}} \right) = 58.2/\left( {M = \frac{{mRT}}{{PV}}} \right) = 58.3\);
\({{\text{C}}_4}{{\text{H}}_{10}}\);
Br2/bromine ;
UV/ultraviolet light;
Accept hf/hv/sunlight.
initiation:
\({\text{B}}{{\text{r}}_2} \to {\text{2Br}} \bullet \);
propagation:
\({\text{Br}} \bullet + {\text{RC}}{{\text{H}}_3} \to {\text{HBr}} + {\text{RC}}{{\text{H}}_2} \bullet \);
\({\text{RC}}{{\text{H}}_2} \bullet + {\text{B}}{{\text{r}}_2} \to {\text{RC}}{{\text{H}}_2}{\text{Br}} + {\text{Br}} \bullet \);
termination: [1 max]
\({\text{Br}} \bullet + {\text{Br}} \bullet \to {\text{B}}{{\text{r}}_2}\);
\({\text{RC}}{{\text{H}}_2} \bullet + {\text{Br}} \bullet \to {\text{RC}}{{\text{H}}_2}{\text{Br}}\);
\({\text{RC}}{{\text{H}}_2} \bullet + {\text{RC}}{{\text{H}}_2} \bullet \to {\text{RC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{R}}\);
Award [1] for any termination step.
Accept radical with or without throughout.
Do not penalise the use of an incorrect alkane in the mechanism.
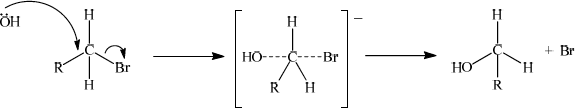
curly arrow going from lone pair/negative charge on O in OH– to C;
Do not allow curly arrow originating on H in OH–.
curly arrow showing Br leaving;
Accept curly arrow either going from bond between C and Br to Br in bromoethane or in the transition state.
representation of transition state showing negative charge, square brackets and partial bonds;
Do not penalize if HO and Br are not at 180° to each other.
Do not award M3 if OH ---- C bond is represented unless already penalised in M1.
Do not penalise the use of an incorrect alkyl chain in the mechanism.
 ;
;
 ;
;
First and second structures should be mirror images. Tetrahedral arrangement around carbon must be shown.
(i) order with respect to \({\text{O}}{{\text{H}}^ - } = {\text{0}}\);
order with respect to \({\text{X}} = 1\);
rate \( = k{\text{[X]}}\);
Award [3] for final correct answer.
(ii) 0.2(0);
\({\text{mi}}{{\text{n}}^{ - 1}}\);
(iii) 2-bromo-2-methyl-propane;
Do not penalize missing hyphens or added spaces.
Accept 2-methyl-2-bromopropane.
tertiary structure;
(iv) \({{\text{C}}_4}{{\text{H}}_9}{\text{Br}} \to {{\text{C}}_4}{\text{H}}_9^ + + {\text{B}}{{\text{r}}^ - }\) / in equation with curly arrows and slow;
\({{\text{C}}_4}{\text{H}}_9^ + + {\text{O}}{{\text{H}}^ - } \to {{\text{C}}_4}{{\text{H}}_9}{\text{OH}}\) / in equation with curly arrows and fast;
No penalty if primary structure is shown.
No credit for SN2 mechanism, except by ECF.
Examiners report
Although this was the least popular question in Section B there was generally a good level of performance. In (a) most candidates scored at least 2 out of 3 marks for calculating the empirical formula.
Many managed to give a correct molecular formula based on their background knowledge once they had determined the molar mass from the density calculation.
The conditions of free radical substitution were well known.
The mechanism of free radical substitution was well known.
The conditions and mechanism of free radical substitution were well known but the SN2 mechanism in (e) caused more problems.
Again the use of curly arrows proved to be difficult. In some case they originated from the H not the lone pair on O of the nucelophile, or were missing from the C – Br bond. Another common mistake was the omission of a negative charge from the transition state. As the attack of the nucleophile is on the opposite side of the carbon atom to the halogen leaving, the partial bonds in the transition state should be drawn at 180 degrees. Candidates were not penalised however if they failed to do this.
Most candidates were able to draw accurate 3D diagrams for the stereoisomers of 2-bromobutane, to deduce the rate expression from the experimental data presented in (g), and correctly identify X as having a tertiary structure. It was also pleasing to see that most were able to describe the SN1 mechanism.
The reactivity of organic compounds depends on the nature and positions of their functional groups.
The structural formulas of two organic compounds are shown below.
Deduce, giving a reason, which of the two compounds can show optical activity.
Draw three-dimensional representations of the two enantiomers.
State the reagents used in the nitration of benzene.
State an equation for the formation of NO2+.
Explain the mechanism of the reaction between 2-bromo-2-methylpropane, (CH3)3CBr, and aqueous sodium hydroxide, NaOH (aq), using curly arrows to represent the movement of electron pairs.
Markscheme
A AND it has a chiral centre/asymmetric carbon atom/carbon with 4 different substituents
Accept structures without tapered bonds.
concentrated HNO3 AND concentrated H2SO4
“concentrated” must occur at least once (with either acid).
HNO3 + 2H2SO4 \( \rightleftharpoons \) H3O+ + NO2+ + 2HSO4–
Accept: HNO3 + 2H2SO4 \( \rightleftharpoons \) NO2+ + HSO4– + H2O.
Accept: HNO3 + 2H2SO4 \( \rightleftharpoons \) H2NO3+ + HSO4–.
Accept single arrow instead of equilibrium sign.
Accept equivalent two step reactions in which sulfuric acid first behaves as strong acid and protonates nitric acid, before behaving as dehydrating agent removing water from it.
curly arrow showing Br– leaving
representation of tertiary carbocation
curly arrow going from lone pair/negative charge on O in –OH to C+
formation of (CH3)3COH AND Br–
Do not accept curly arrow originating from C of C–Br bond.
Do not accept arrow originating on H in –OH.
Accept Br– anywhere on product side in the reaction scheme.
Award [2 max] for an SN2 type mechanism.
Examiners report
Organic compounds often have isomers.
A straight chain molecule of formula C5H10O contains a carbonyl group. The compound cannot be oxidized by acidified potassium dichromate(VI) solution.
A tertiary halogenoalkane with three different alkyl groups, (R1R2R3)C−X, undergoes a SN1 reaction and forms two isomers.
Deduce the structural formulas of the two possible isomers.
Mass spectra A and B of the two isomers are given.
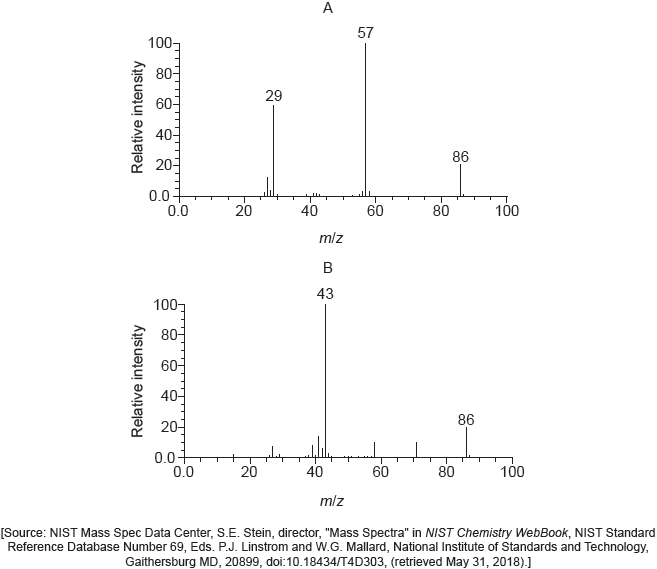
Explain which spectrum is produced by each compound using section 28 of the data booklet.
State the type of bond fission that takes place in a SN1 reaction.
State the type of solvent most suitable for the reaction.
Draw the structure of the intermediate formed stating its shape.
Suggest, giving a reason, the percentage of each isomer from the SN1 reaction.
Nitrobenzene, C6H5NO2, can be converted to phenylamine via a two-stage reaction.
In the first stage, nitrobenzene is reduced with tin in an acidic solution to form an intermediate ion and tin(II) ions. In the second stage, the intermediate ion is converted to phenylamine in the presence of hydroxide ions.
Formulate the equation for each stage of the reaction.
Markscheme
Accept condensed formulas.
[2 marks]
A:
CH3CH2COCH2CH3 AND «peak at» 29 due to
(CH3CH2)+/(C2H5)+/(M – CH3CH2CO)+
OR
CH3CH2COCH2CH3 AND «peak at» 57 due to
(CH3CH2CO)+/(M – CH3CH2)+/(M – C2H5)+
B:
CH3COCH2CH2CH3 AND «peak at» 43 due to
(CH3CH2CH2)+/(CH3CO)+/(C2H3O)+/(M – CH3CO)+
Penalize missing “+” sign once only.
Accept “CH3COCH2CH2CH3 by elimination since fragment CH3CO is not listed” for M2.
[2 marks]
heterolytic/heterolysis
[1 mark]
polar protic
[1 mark]
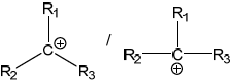
Shape: triangular/trigonal planar
[2 marks]
«around» 50% «each»
OR
similar/equal percentages
nucleophile can attack from either side «of the planar carbocation»
Accept “racemic mixture/racemate” for M1.
[2 marks]
Stage one:
C6H5NO2(l) + 3Sn(s) + 7H+(aq) → C6H5NH3+(aq) + 3Sn2+(aq) + 2H2O(l)
Stage two:
C6H5NH3+(aq) + OH–(aq) → C6H5NH2(l) + H2O(l)
[2 marks]
Examiners report
Benzene is an aromatic hydrocarbon.
Discuss the physical evidence for the structure of benzene.
State the typical reactions that benzene and cyclohexene undergo with bromine.
State the reagents used to convert benzene to nitrobenzene and the formula of the electrophile formed.
Explain the mechanism for the nitration of benzene, using curly arrows to show the movement of electron pairs.
State the reagents used in the two-stage conversion of nitrobenzene to aniline.
Markscheme
Any two of:
planar «X-ray»
C to C bond lengths all equal
OR
C to C bonds intermediate in length between C–C and C=C
all C–C–C bond angles equal
[2 marks]
benzene: «electrophilic» substitution/SE
AND
cyclohexene: «electrophilic» addition/AE
Accept correct equations.
[1 mark]
«concentrated» nitric AND sulfuric acids
+NO2
Accept NO2+.
[2 marks]
curly arrow going from benzene ring to N of +NO2/NO2+
carbocation with correct formula and positive charge on ring
curly arrow going from C–H bond to benzene ring of cation
formation of organic product AND H+
Accept mechanism with corresponding Kekulé structures.
Do not accept a circle in M2 or M3.
Accept first arrow starting either inside the circle or on the circle.
M2 may be awarded from correct diagram for M3.
M4: Accept C6H5NO2 + H2SO4 if HSO4– used in M3.
Fe/Zn/Sn AND HCl/H2SO4/CH3COOH
NaOH/KOH
Accept other suitable metals and acids.
Accept other suitable bases.
Award [1 max] for single-step reducing agents (such as H2/Pt, Na2S etc.).
Accept formulas or names.
[2 marks]
Examiners report
Bromomethane was used as a pesticide until it was found to be ozone-depleting.
State the equation for the reaction between methane and bromine to form bromomethane.
Explain, using equations, the complete free-radical mechanism for the reaction of methane with bromine, including necessary reaction conditions.
Bromomethane reacts with aqueous sodium hydroxide. State the organic product of this reaction.
Explain why the rate of the reaction between iodomethane, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{I}}\), and NaOH(aq) is faster than the rate of the reaction between \({\text{C}}{{\text{H}}_{\text{3}}}{\text{Br}}\) and NaOH(aq).
Bromine can be produced by the electrolysis of molten sodium bromide.
Deduce the half-equation for the reaction at each electrode.
Positive electrode (anode):
Negative electrode (cathode):
Predict the products formed at the electrodes during the electrolysis of concentrated aqueous sodium bromide.
Positive electrode (anode):
Negative electrode (cathode):
Bromine reacts with aqueous sodium iodide.
\[{\text{B}}{{\text{r}}_{\text{2}}}{\text{(aq)}} + {\text{2NaI(aq)}} \to {{\text{I}}_{\text{2}}}{\text{(aq)}} + {\text{2NaBr(aq)}}\]
Identify the oxidizing agent in this reaction.
Define the term standard electrode potential, \({E^\Theta }\).
Draw a labelled diagram for the voltaic cell in which the following reaction occurs.
\[{\text{Mg(s)}} + {\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}} \to {\text{M}}{{\text{g}}^{2 + }}{\text{(aq)}} + {\text{Cu(s)}}\]
Include in your answer the direction of electron flow and the polarity of the electrodes.
A student measures a voltage of 2.65 V in the voltaic cell formed between magnesium and copper half-cells using a digital voltmeter.
State the random uncertainty of this value, in V, and the number of significant figures in the answer.
Random uncertainty:
Significant figures:
Outline how the student can reduce the random error in her results.
Determine the standard enthalpy change of formation, \(\Delta H_{\text{f}}^\Theta \), of NaCl(s), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), using a Born-Haber cycle and tables 7, 10 and 13 of the data booklet. The standard enthalpy change of atomization (standard enthalpy change of sublimation), \(\Delta H_{{\text{at}}}^\Theta \), of Na(s) is \( + {\text{108 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
Markscheme
\({\text{C}}{{\text{H}}_{\text{4}}} + {\text{B}}{{\text{r}}_{\text{2}}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{Br}} + {\text{HBr}}\);
Initiation:
\({\text{B}}{{\text{r}}_2}\xrightarrow{{{\text{UV/}}hf{\text{/}}hv}}{\text{2Br}} \bullet \);
Reference to UV/light or high temperatures must be included.
Propagation:
\({\text{Br}} \bullet + {\text{C}}{{\text{H}}_4} \to {\text{C}}{{\text{H}}_3} \bullet + {\text{HBr}}\);
\({\text{C}}{{\text{H}}_3} \bullet + {\text{B}}{{\text{r}}_2} \to {\text{C}}{{\text{H}}_3}{\text{Br}} + {\text{Br}} \bullet \);
Termination:
Award [1 max] for any one of:
\({\text{Br}} \bullet + {\text{Br}} \bullet \to {\text{B}}{{\text{r}}_2}\);
\({\text{C}}{{\text{H}}_3} \bullet + {\text{Br}} \bullet \to {\text{C}}{{\text{H}}_3}{\text{Br}}\);
\({\text{C}}{{\text{H}}_3} \bullet + {\text{C}}{{\text{H}}_3} \bullet \to {{\text{C}}_2}{{\text{H}}_6}\);
Allow representation of radical without \( \bullet \) (eg Br, \(C{H_{\text{3}}}\)) if consistent throughout mechanism.
Award [3 max] if initiation, propagation and termination are not stated or are incorrectly labelled for equations.
\({\text{methanol/C}}{{\text{H}}_{\text{3}}}{\text{OH}}\);
C–I bond is weaker than the C–Br bond so more easily broken;
C–I bond is longer than the C–Br bond / I larger than Br so bonding electrons not as tightly held / \({{\text{I}}^ - }\) is better leaving group than \({\text{B}}{{\text{r}}^ - }\);
Positive electrode (anode):
\({\text{2B}}{{\text{r}}^ - } \to {\text{B}}{{\text{r}}_2}{\text{(g)}} + {\text{2}}{{\text{e}}^ - }{\text{/B}}{{\text{r}}^ - } \to \frac{1}{2}{\text{B}}{{\text{r}}_2}{\text{(g)}} + {{\text{e}}^ - }\);
Negative electrode (cathode):
\({\text{N}}{{\text{a}}^ + } + {{\text{e}}^ - } \to {\text{Na(l)}}\);
Award [1 max] for correct equations at incorrect electrodes.
Ignore state symbols.
Accept e instead of \({{\text{e}}^ - }\).
Penalize use of equilibrium signs once only.
Positive electrode (anode):
bromine/\({\text{B}}{{\text{r}}_{\text{2}}}\);
Negative electrode (cathode):
hydrogen/\({{\text{H}}_{\text{2}}}\);
Allow sodium hydroxide/NaOH/hydroxide/\(O{H^ - }\) formation.
bromine/\({\text{B}}{{\text{r}}_{\text{2}}}\);
Do not accept bromide/\(B{r^ - }\).
potential of reduction half-reaction under standard conditions measured relative to standard hydrogen electrode/SHE/potential under standard conditions relative to standard hydrogen electrode/SHE;
Instead of standard state allow either solute concentration of \(1 mol\,d{m^{ - {\text{3}}}}\) or
\(100 kPa/1.00 \times 1{0^5} Pa\) for gases.
Allow 1 bar for \(100 kPa/1.00 \times 1{0^5} Pa\).
Allow 1 atm.
Allow voltage instead of potential.
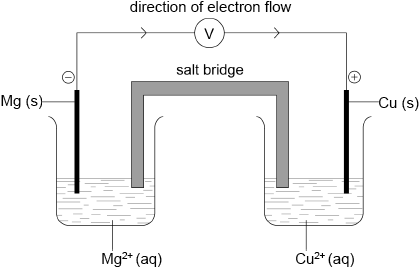
correct diagram including (voltmeter), 4 correct species (state symbols not required) and connecting wires;
No credit if wires to electrodes immersed in the solutions.
Accept ammeter/meter/lamp instead of voltmeter.
labelled salt bridge;
Accept an appropriate salt (name or formula) instead of salt bridge (eg, potassium nitrate).
correctly labelled electrodes as +/cathode and −/anode;
flow of electrons from Mg to Cu in external circuit;
Random uncertainty: (±) 0.01 (V);
Significant figures: 3;
repeat readings and take an average / use more precise equipment;
\({\text{atomization of chlorine}} = \frac{1}{2}{\text{ bond enthalpy / }}\frac{1}{2}{\text{ 243 / 121.5 (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
correct values for ionization Na (\( + {\text{496 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)) and electron affinity Cl (\( - {\text{349 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\))
and lattice enthalpy of NaCl (\( + {\text{790 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{ /}} + {\text{769 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\));
Born-Haber energy cycle;
Accept lines or arrows in energy cycle.

\(\Delta H_{\text{f}}^\Theta {\text{(NaCl(s))}} = - {\text{413.5 /}} - {\text{413 /}} - {\text{414 (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Accept \( - 392.5 / - 392 / - 393 if + 769\) used for lattice enthalpy.
Award [4] for correct final answer.
Examiners report
Candidates found it difficult to write the equation in (a) and the mechanisms in (b) (i) ranged from really good to no understanding. Many opined the production of \( \bullet {\text{H}}\) in the first propagation step. A significant number of candidates suggested a mechanism involving ions despite free radical begin stated in the stem. Most were able to give methanol in (ii). Few scored full marks for (c); the answer needed to be thought through carefully. In (d) the electrodes were often reversed or the equations unbalanced. Few understood the significance of the water present in the answers to (ii). A high percentage of candidates gave the correct answer to (e) but (f) was poorly answered. Either the standard hydrogen electrode or standard conditions were omitted in (i) and the standard of diagrams in (ii) was very poor indeed. Little care seemed to have been taken over their presentation; it was not clear what, if anything, was in the beakers and electrode connections were shown actually in the solutions. In (iii) some did not notice that the voltmeter was digital but most gave the number of significant figures correctly. In (iv) many suggested repeated readings but few stated that an average omitted must be taken. In (g), those who didn’t draw out the cycle tended to get the answer wrong. Examiners cannot give part marks if they cannot work out what is being done. There was one mark for a correct Born-Haber cycle. Very few gained the mark for dividing the chlorine value by 2.
Candidates found it difficult to write the equation in (a) and the mechanisms in (b) (i) ranged from really good to no understanding. Many opined the production of \( \bullet {\text{H}}\) in the first propagation step. A significant number of candidates suggested a mechanism involving ions despite free radical begin stated in the stem. Most were able to give methanol in (ii). Few scored full marks for (c); the answer needed to be thought through carefully. In (d) the electrodes were often reversed or the equations unbalanced. Few understood the significance of the water present in the answers to (ii). A high percentage of candidates gave the correct answer to (e) but (f) was poorly answered. Either the standard hydrogen electrode or standard conditions were omitted in (i) and the standard of diagrams in (ii) was very poor indeed. Little care seemed to have been taken over their presentation; it was not clear what, if anything, was in the beakers and electrode connections were shown actually in the solutions. In (iii) some did not notice that the voltmeter was digital but most gave the number of significant figures correctly. In (iv) many suggested repeated readings but few stated that an average omitted must be taken. In (g), those who didn’t draw out the cycle tended to get the answer wrong. Examiners cannot give part marks if they cannot work out what is being done. There was one mark for a correct Born-Haber cycle. Very few gained the mark for dividing the chlorine value by 2.
Candidates found it difficult to write the equation in (a) and the mechanisms in (b) (i) ranged from really good to no understanding. Many opined the production of \( \bullet {\text{H}}\) in the first propagation step. A significant number of candidates suggested a mechanism involving ions despite free radical begin stated in the stem. Most were able to give methanol in (ii). Few scored full marks for (c); the answer needed to be thought through carefully. In (d) the electrodes were often reversed or the equations unbalanced. Few understood the significance of the water present in the answers to (ii). A high percentage of candidates gave the correct answer to (e) but (f) was poorly answered. Either the standard hydrogen electrode or standard conditions were omitted in (i) and the standard of diagrams in (ii) was very poor indeed. Little care seemed to have been taken over their presentation; it was not clear what, if anything, was in the beakers and electrode connections were shown actually in the solutions. In (iii) some did not notice that the voltmeter was digital but most gave the number of significant figures correctly. In (iv) many suggested repeated readings but few stated that an average omitted must be taken. In (g), those who didn’t draw out the cycle tended to get the answer wrong. Examiners cannot give part marks if they cannot work out what is being done. There was one mark for a correct Born-Haber cycle. Very few gained the mark for dividing the chlorine value by 2.
Candidates found it difficult to write the equation in (a) and the mechanisms in (b) (i) ranged from really good to no understanding. Many opined the production of \( \bullet {\text{H}}\) in the first propagation step. A significant number of candidates suggested a mechanism involving ions despite free radical begin stated in the stem. Most were able to give methanol in (ii). Few scored full marks for (c); the answer needed to be thought through carefully. In (d) the electrodes were often reversed or the equations unbalanced. Few understood the significance of the water present in the answers to (ii). A high percentage of candidates gave the correct answer to (e) but (f) was poorly answered. Either the standard hydrogen electrode or standard conditions were omitted in (i) and the standard of diagrams in (ii) was very poor indeed. Little care seemed to have been taken over their presentation; it was not clear what, if anything, was in the beakers and electrode connections were shown actually in the solutions. In (iii) some did not notice that the voltmeter was digital but most gave the number of significant figures correctly. In (iv) many suggested repeated readings but few stated that an average omitted must be taken. In (g), those who didn’t draw out the cycle tended to get the answer wrong. Examiners cannot give part marks if they cannot work out what is being done. There was one mark for a correct Born-Haber cycle. Very few gained the mark for dividing the chlorine value by 2.
Candidates found it difficult to write the equation in (a) and the mechanisms in (b) (i) ranged from really good to no understanding. Many opined the production of \( \bullet {\text{H}}\) in the first propagation step. A significant number of candidates suggested a mechanism involving ions despite free radical begin stated in the stem. Most were able to give methanol in (ii). Few scored full marks for (c); the answer needed to be thought through carefully. In (d) the electrodes were often reversed or the equations unbalanced. Few understood the significance of the water present in the answers to (ii). A high percentage of candidates gave the correct answer to (e) but (f) was poorly answered. Either the standard hydrogen electrode or standard conditions were omitted in (i) and the standard of diagrams in (ii) was very poor indeed. Little care seemed to have been taken over their presentation; it was not clear what, if anything, was in the beakers and electrode connections were shown actually in the solutions. In (iii) some did not notice that the voltmeter was digital but most gave the number of significant figures correctly. In (iv) many suggested repeated readings but few stated that an average omitted must be taken. In (g), those who didn’t draw out the cycle tended to get the answer wrong. Examiners cannot give part marks if they cannot work out what is being done. There was one mark for a correct Born-Haber cycle. Very few gained the mark for dividing the chlorine value by 2.
Candidates found it difficult to write the equation in (a) and the mechanisms in (b) (i) ranged from really good to no understanding. Many opined the production of \( \bullet {\text{H}}\) in the first propagation step. A significant number of candidates suggested a mechanism involving ions despite free radical begin stated in the stem. Most were able to give methanol in (ii). Few scored full marks for (c); the answer needed to be thought through carefully. In (d) the electrodes were often reversed or the equations unbalanced. Few understood the significance of the water present in the answers to (ii). A high percentage of candidates gave the correct answer to (e) but (f) was poorly answered. Either the standard hydrogen electrode or standard conditions were omitted in (i) and the standard of diagrams in (ii) was very poor indeed. Little care seemed to have been taken over their presentation; it was not clear what, if anything, was in the beakers and electrode connections were shown actually in the solutions. In (iii) some did not notice that the voltmeter was digital but most gave the number of significant figures correctly. In (iv) many suggested repeated readings but few stated that an average omitted must be taken. In (g), those who didn’t draw out the cycle tended to get the answer wrong. Examiners cannot give part marks if they cannot work out what is being done. There was one mark for a correct Born-Haber cycle. Very few gained the mark for dividing the chlorine value by 2.
Candidates found it difficult to write the equation in (a) and the mechanisms in (b) (i) ranged from really good to no understanding. Many opined the production of \( \bullet {\text{H}}\) in the first propagation step. A significant number of candidates suggested a mechanism involving ions despite free radical begin stated in the stem. Most were able to give methanol in (ii). Few scored full marks for (c); the answer needed to be thought through carefully. In (d) the electrodes were often reversed or the equations unbalanced. Few understood the significance of the water present in the answers to (ii). A high percentage of candidates gave the correct answer to (e) but (f) was poorly answered. Either the standard hydrogen electrode or standard conditions were omitted in (i) and the standard of diagrams in (ii) was very poor indeed. Little care seemed to have been taken over their presentation; it was not clear what, if anything, was in the beakers and electrode connections were shown actually in the solutions. In (iii) some did not notice that the voltmeter was digital but most gave the number of significant figures correctly. In (iv) many suggested repeated readings but few stated that an average omitted must be taken. In (g), those who didn’t draw out the cycle tended to get the answer wrong. Examiners cannot give part marks if they cannot work out what is being done. There was one mark for a correct Born-Haber cycle. Very few gained the mark for dividing the chlorine value by 2.
Candidates found it difficult to write the equation in (a) and the mechanisms in (b) (i) ranged from really good to no understanding. Many opined the production of \( \bullet {\text{H}}\) in the first propagation step. A significant number of candidates suggested a mechanism involving ions despite free radical begin stated in the stem. Most were able to give methanol in (ii). Few scored full marks for (c); the answer needed to be thought through carefully. In (d) the electrodes were often reversed or the equations unbalanced. Few understood the significance of the water present in the answers to (ii). A high percentage of candidates gave the correct answer to (e) but (f) was poorly answered. Either the standard hydrogen electrode or standard conditions were omitted in (i) and the standard of diagrams in (ii) was very poor indeed. Little care seemed to have been taken over their presentation; it was not clear what, if anything, was in the beakers and electrode connections were shown actually in the solutions. In (iii) some did not notice that the voltmeter was digital but most gave the number of significant figures correctly. In (iv) many suggested repeated readings but few stated that an average omitted must be taken. In (g), those who didn’t draw out the cycle tended to get the answer wrong. Examiners cannot give part marks if they cannot work out what is being done. There was one mark for a correct Born-Haber cycle. Very few gained the mark for dividing the chlorine value by 2.
Candidates found it difficult to write the equation in (a) and the mechanisms in (b) (i) ranged from really good to no understanding. Many opined the production of \( \bullet {\text{H}}\) in the first propagation step. A significant number of candidates suggested a mechanism involving ions despite free radical begin stated in the stem. Most were able to give methanol in (ii). Few scored full marks for (c); the answer needed to be thought through carefully. In (d) the electrodes were often reversed or the equations unbalanced. Few understood the significance of the water present in the answers to (ii). A high percentage of candidates gave the correct answer to (e) but (f) was poorly answered. Either the standard hydrogen electrode or standard conditions were omitted in (i) and the standard of diagrams in (ii) was very poor indeed. Little care seemed to have been taken over their presentation; it was not clear what, if anything, was in the beakers and electrode connections were shown actually in the solutions. In (iii) some did not notice that the voltmeter was digital but most gave the number of significant figures correctly. In (iv) many suggested repeated readings but few stated that an average omitted must be taken. In (g), those who didn’t draw out the cycle tended to get the answer wrong. Examiners cannot give part marks if they cannot work out what is being done. There was one mark for a correct Born-Haber cycle. Very few gained the mark for dividing the chlorine value by 2.
Candidates found it difficult to write the equation in (a) and the mechanisms in (b) (i) ranged from really good to no understanding. Many opined the production of \( \bullet {\text{H}}\) in the first propagation step. A significant number of candidates suggested a mechanism involving ions despite free radical begin stated in the stem. Most were able to give methanol in (ii). Few scored full marks for (c); the answer needed to be thought through carefully. In (d) the electrodes were often reversed or the equations unbalanced. Few understood the significance of the water present in the answers to (ii). A high percentage of candidates gave the correct answer to (e) but (f) was poorly answered. Either the standard hydrogen electrode or standard conditions were omitted in (i) and the standard of diagrams in (ii) was very poor indeed. Little care seemed to have been taken over their presentation; it was not clear what, if anything, was in the beakers and electrode connections were shown actually in the solutions. In (iii) some did not notice that the voltmeter was digital but most gave the number of significant figures correctly. In (iv) many suggested repeated readings but few stated that an average omitted must be taken. In (g), those who didn’t draw out the cycle tended to get the answer wrong. Examiners cannot give part marks if they cannot work out what is being done. There was one mark for a correct Born-Haber cycle. Very few gained the mark for dividing the chlorine value by 2.
Candidates found it difficult to write the equation in (a) and the mechanisms in (b) (i) ranged from really good to no understanding. Many opined the production of \( \bullet {\text{H}}\) in the first propagation step. A significant number of candidates suggested a mechanism involving ions despite free radical begin stated in the stem. Most were able to give methanol in (ii). Few scored full marks for (c); the answer needed to be thought through carefully. In (d) the electrodes were often reversed or the equations unbalanced. Few understood the significance of the water present in the answers to (ii). A high percentage of candidates gave the correct answer to (e) but (f) was poorly answered. Either the standard hydrogen electrode or standard conditions were omitted in (i) and the standard of diagrams in (ii) was very poor indeed. Little care seemed to have been taken over their presentation; it was not clear what, if anything, was in the beakers and electrode connections were shown actually in the solutions. In (iii) some did not notice that the voltmeter was digital but most gave the number of significant figures correctly. In (iv) many suggested repeated readings but few stated that an average omitted must be taken. In (g), those who didn’t draw out the cycle tended to get the answer wrong. Examiners cannot give part marks if they cannot work out what is being done. There was one mark for a correct Born-Haber cycle. Very few gained the mark for dividing the chlorine value by 2.
Candidates found it difficult to write the equation in (a) and the mechanisms in (b) (i) ranged from really good to no understanding. Many opined the production of \( \bullet {\text{H}}\) in the first propagation step. A significant number of candidates suggested a mechanism involving ions despite free radical begin stated in the stem. Most were able to give methanol in (ii). Few scored full marks for (c); the answer needed to be thought through carefully. In (d) the electrodes were often reversed or the equations unbalanced. Few understood the significance of the water present in the answers to (ii). A high percentage of candidates gave the correct answer to (e) but (f) was poorly answered. Either the standard hydrogen electrode or standard conditions were omitted in (i) and the standard of diagrams in (ii) was very poor indeed. Little care seemed to have been taken over their presentation; it was not clear what, if anything, was in the beakers and electrode connections were shown actually in the solutions. In (iii) some did not notice that the voltmeter was digital but most gave the number of significant figures correctly. In (iv) many suggested repeated readings but few stated that an average omitted must be taken. In (g), those who didn’t draw out the cycle tended to get the answer wrong. Examiners cannot give part marks if they cannot work out what is being done. There was one mark for a correct Born-Haber cycle. Very few gained the mark for dividing the chlorine value by 2.
\({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ethanoic acid was added to \({\text{30.0 c}}{{\text{m}}^{\text{3}}}\) of a \({\text{0.150 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydrogencarbonate solution, \({\text{NaHC}}{{\text{O}}_{\text{3}}}{\text{(aq)}}\).
The molar mass of a volatile organic liquid, X, can be determined experimentally by allowing it to vaporize completely at a controlled temperature and pressure. 0.348 g of X was injected into a gas syringe maintained at a temperature of 90 °C and a pressure of \(1.01 \times {10^5}{\text{ Pa}}\). Once it had reached equilibrium, the gas volume was measured as \({\text{95.0 c}}{{\text{m}}^{\text{3}}}\).
Bromoethane, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Br}}\), undergoes a substitution reaction to form ethylamine, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{N}}{{\text{H}}_{\text{2}}}\).
Many organic compounds exist as stereoisomers.
Outline how electrical conductivity can be used to distinguish between a \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of ethanoic acid, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\), and a \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of hydrochloric acid, HCl.
(i) State an equation for the reaction of ethanoic acid with a solution of sodium hydrogencarbonate.
(ii) Determine which is the limiting reagent. Show your working.
(iii) Calculate the mass, in g, of carbon dioxide gas produced.
(i) Determine the amount, in mol, of X in the gas syringe.
(ii) Calculate the molar mass of X.
Deduce the mechanism for the reaction using equations and curly arrows to represent the movement of electron pairs.
(i) Outline the meaning of the term stereoisomers.
(ii) Draw the structures of the two stereoisomers of dichloroethene, \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\).
(iii) Explain why this type of stereoisomerism exists in \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\).
(iv) Draw the structures of the two stereoisomers of 1-chloro-1-fluoroethane, \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{FCl}}\), showing the relationship between them.
(v) Outline how the two isomers of \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{FCl}}\) could be distinguished from each other.
Markscheme
HCl is a strong acid and \({\text{C}}{{\text{H}}_3}{\text{COOH}}\) is a weak acid so HCl has higher conductivity / HCl dissociates completely in water and \({\text{C}}{{\text{H}}_3}{\text{COOH}}\) does not, so HCl has higher conductivity / HCl is a stronger acid (than \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) so has higher \({\text{[}}{{\text{H}}^ + }{\text{]}}\) and higher conductivity;
(i) \({\text{C}}{{\text{H}}_3}{\text{COOH(aq)}} + {\text{HCO}}_3^ - {\text{(aq)}} \to {\text{C}}{{\text{H}}_3}{\text{CO}}{{\text{O}}^ - }{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}}\);
Accept NaHCO3(aq) and CH3COONa (aq) instead of ions.
Ignore state symbols.
(ii) \(n({\text{C}}{{\text{H}}_3}{\text{COOH}}) = 0.00500{\text{ (mol)}}\) and \(n{\text{(NaHC}}{{\text{O}}_3}{\text{)}} = 0.00450{\text{ (mol)}}\);
\({\text{NaHC}}{{\text{O}}_3}\) is limiting;
(iii) \(n{\text{(C}}{{\text{O}}_2}{\text{)}} = n{\text{(NaHC}}{{\text{O}}_{\text{3}}}{\text{)}} = 0.00450{\text{ (mol)}}\);
\(m{\text{(C}}{{\text{O}}_2}{\text{)}} = 0.00450 \times 44.01 = 0.198{\text{(g)}}\);
Award [2] for correct final answer.
(i) \(T = 363{\text{ K}}\) and \(V = 9.50 \times {10^{ - 5}}{\text{ }}{{\text{m}}^3}\);
Accept V = 9.5 \( \times \) 10–2 dm3 if P is used as 101 kPa in calculation.
\(n = \frac{{PV}}{{RT}} = \frac{{1.01 \times {{10}^5} \times 9.50 \times {{10}^{ - 5}}}}{{8.31 \times 363}}\);
\( = 3.18 \times {10^{ - 3}}{\text{ (mol)}}\);
Award [3] for correct final answer.
(ii) \(M - \left( {\frac{m}{n} = \frac{{0.348}}{{3.18 \times {{10}^{ - 3}}}} = } \right){\text{ }}109{\text{ (g}}\,{\text{mo}}{{\text{l}}^{ - {\text{1}}}}{\text{)}}\);
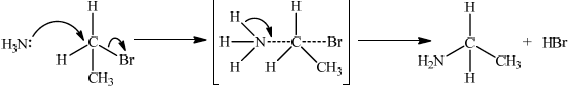
curly arrow going from lone pair on N in \({\text{N}}{{\text{H}}_{\text{3}}}\) to C;
curly arrow showing Br leaving;
Accept curly arrow going from bond between C and Br to Br on 1-bromoethane or on the transition state.
representation of transition state showing square brackets, two partial bonds and curly arrow going from NH bond to NC partial bond/curly arrow going from NH bond to N;
Do not penalize if NH3 and Br are not at 180° to each other.
Do not award M3 if NH3—C bond is represented.
(i) compounds with same structural formula but different arrangements of atoms in space;
(ii)
The two structures must be clear 3D representations of mirror images.
Tapered (wedge/dash) notation not necessary.
(iii) restricted rotation around (C=C) double bond;
(iv)
(v) the two enantiomers rotate the plane of plane-polarized light by equal amounts, but in opposite directions;
using a polarimeter;
Examiners report
Poorly constructed symbolic equations on what should be relatively simple reactions once again impeded candidates from credit. The use of \(pV = nRT\) often scored for error carried forward even when they lost the first mark from incorrect use of units for pressure. The attempts at the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanisms were generally poor, with errors both in the attacking nucleophile, and the sloppy use of curly arrows which indicate that many students have a basic lack of understanding about what they represent. While candidates could score the first two marks, the third mark was almost never awarded. Conditions and reagents in d(ii) and d(iii) were rarely known, and definitions of stereoisomers and the representation of 3D structures was disappointing.
Poorly constructed symbolic equations on what should be relatively simple reactions once again impeded candidates from credit. The use of \(pV = nRT\) often scored for error carried forward even when they lost the first mark from incorrect use of units for pressure. The attempts at the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanisms were generally poor, with errors both in the attacking nucleophile, and the sloppy use of curly arrows which indicate that many students have a basic lack of understanding about what they represent. While candidates could score the first two marks, the third mark was almost never awarded. Conditions and reagents in d(ii) and d(iii) were rarely known, and definitions of stereoisomers and the representation of 3D structures was disappointing.
Poorly constructed symbolic equations on what should be relatively simple reactions once again impeded candidates from credit. The use of \(pV = nRT\) often scored for error carried forward even when they lost the first mark from incorrect use of units for pressure. The attempts at the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanisms were generally poor, with errors both in the attacking nucleophile, and the sloppy use of curly arrows which indicate that many students have a basic lack of understanding about what they represent. While candidates could score the first two marks, the third mark was almost never awarded. Conditions and reagents in d(ii) and d(iii) were rarely known, and definitions of stereoisomers and the representation of 3D structures was disappointing.
Poorly constructed symbolic equations on what should be relatively simple reactions once again impeded candidates from credit. The use of \(pV = nRT\) often scored for error carried forward even when they lost the first mark from incorrect use of units for pressure. The attempts at the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanisms were generally poor, with errors both in the attacking nucleophile, and the sloppy use of curly arrows which indicate that many students have a basic lack of understanding about what they represent. While candidates could score the first two marks, the third mark was almost never awarded. Conditions and reagents in d(ii) and d(iii) were rarely known, and definitions of stereoisomers and the representation of 3D structures was disappointing.
Poorly constructed symbolic equations on what should be relatively simple reactions once again impeded candidates from credit. The use of \(pV = nRT\) often scored for error carried forward even when they lost the first mark from incorrect use of units for pressure. The attempts at the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanisms were generally poor, with errors both in the attacking nucleophile, and the sloppy use of curly arrows which indicate that many students have a basic lack of understanding about what they represent. While candidates could score the first two marks, the third mark was almost never awarded. Conditions and reagents in d(ii) and d(iii) were rarely known, and definitions of stereoisomers and the representation of 3D structures was disappointing.
This question is about carbon and chlorine compounds.
Ethane, \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}\), reacts with chlorine in sunlight. State the type of this reaction and the name of the mechanism by which it occurs.
Formulate equations for the two propagation steps and one termination step in the formation of chloroethane from ethane.
Deduce the splitting patterns in the 1H NMR spectrum of C2H5Cl.
Explain why tetramethylsilane (TMS) is often used as a reference standard in 1H NMR.
One possible product, X, of the reaction of ethane with chlorine has the following composition by mass:
carbon: 24.27%, hydrogen: 4.08%, chlorine: 71.65%
Determine the empirical formula of the product.
The mass and 1H NMR spectra of product X are shown below. Deduce, giving your reasons, its structural formula and hence the name of the compound.
When the product X is reacted with NaOH in a hot alcoholic solution, C2H3Cl is formed. State the role of the reactant NaOH other than as a nucleophile.
Chloroethene, \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{3}}}{\text{Cl}}\), can undergo polymerization. Draw a section of the polymer with three repeating units.
Markscheme
substitution AND «free-»radical
OR
substitution AND chain
Award [1] for “«free-»radical substitution” or “SR” written anywhere in the answer.
[1 mark]
Two propagation steps:
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}} + \bullet {\text{Cl}} \to {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}} \bullet + {\text{HCl}}\)
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}} \bullet + {\text{C}}{{\text{l}}_{\text{2}}} \to {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{Cl}} + \bullet {\text{Cl}}\)
One termination step:
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}} \bullet + {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}} \bullet \to {{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}\)
OR
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}} \bullet + \bullet {\text{Cl}} \to {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{Cl}}\)
OR
\( \bullet {\text{Cl}} + \bullet {\text{Cl}} \to {\text{C}}{{\text{l}}_{\text{2}}}\)
Accept radical without \( \bullet \) if consistent throughout.
Allow ECF for incorrect radicals produced in propagation step for M3.
[3 marks]
triplet AND quartet
[1 mark]
chemical shift/signal outside range of common chemical shift/signal
strong signal/12/all H atoms in same environment
OR
singlet/no splitting of the signal
volatile/easily separated/easily removed
OR
inert/stabl
contains three common NMR nuclei/1H and 13C and 29Si
Do not accept chemical shift = 0.
[2 marks]
\({\text{C}} = \frac{{24.27}}{{12.01}} = 2.021\) AND \({\text{H}} = \frac{{4.08}}{{1.01}} = 4.04\) AND \({\text{Cl}} = \frac{{71.65}}{{35.45}} = 2.021\)
«hence» CH2Cl
Accept \(\frac{{24.27}}{{12.01}}\) : \(\frac{{4.08}}{{1.01}}\) : \(\frac{{71.65}}{{35.45}}.\)
Do not accept C2H4Cl2.
Award [2] for correct final answer.
[2 marks]
molecular ion peak(s) «about» m/z 100 AND «so» C2H4Cl2 «isotopes of Cl»
two signals «in 1H NMR spectrum» AND «so» CH3CHCl2
OR
«signals in» 3:1 ratio «in 1H NMR spectrum» AND «so» CH3CHCl2
OR
one doublet and one quartet «in 1H NMR spectrum» AND «so» CH3CHCl2
1,1-dichloroethane
Accept “peaks” for “signals”.
Allow ECF for a correct name for M3 if an incorrect chlorohydrocarbon is identified.
[3 marks]
base
OR
proton acceptor
[1 mark]
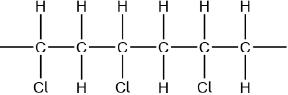
Continuation bonds must be shown.
Ignore square brackets and “n”.
Accept 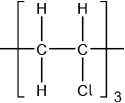 .
.
Accept other versions of the polymer, such as head to head and head to tail.
Accept condensed structure provided all C to C bonds are shown (as single).
[1 mark]
Examiners report
Propane and propene are members of different homologous series.
(i) Draw diagrams to show how sigma (σ) and pi (π) bonds are formed between atoms.
(ii) State the number of sigma (σ) and pi (π) bonds in propane and propene.
Construct the mechanism of the formation of 2-bromopropane from hydrogen bromide and propene using curly arrows to denote the movement of electrons.
Markscheme
i
ii
Award [1] for two or three correct answers.
Award [2] for all four correct.
curly arrow going from C=C to H of HBr and curly arrow showing Br leaving
representation of carbocation
curly arrow going from lone pair/negative charge on Br– to C+
Award [2 max] for formation of 1-bromopropane.
Examiners report
A compound with a molecular formula C7H14O produced the following high resolution 1H NMR spectrum.
Deduce what information can be obtained from the 1H NMR spectrum.
Identify the functional group that shows stretching at 1710 cm–1 in the infrared spectrum of this compound using section 26 of the data booklet and the 1H NMR.
Suggest the structural formula of this compound.
Bromine was added to hexane, hex-1-ene and benzene. Identify the compound(s) which will react with bromine in a well-lit laboratory.
Deduce the structural formula of the main organic product when hex-1-ene reacts with hydrogen bromide.
State the reagents and the name of the mechanism for the nitration of benzene.
Outline, in terms of the bonding present, why the reaction conditions of halogenation are different for alkanes and benzene.
Below are two isomers, A and B, with the molecular formula C4H9Br.
Explain the mechanism of the nucleophilic substitution reaction with NaOH(aq) for the isomer that reacts almost exclusively by an SN2 mechanism using curly arrows to represent the movement of electron pairs.
Markscheme
Number of hydrogen environments: 3
Ratio of hydrogen environments: 2:3:9
Splitting patterns: «all» singlets
Accept any equivalent ratios such as 9:3:2.
Accept “no splitting”.
[3 marks]
carbonyl
OR
C=O
Accept “ketone” but not “aldehyde”.
[1 mark]
Accept (CH3)3CCH2COCH3.
Award [1] for any aldehyde or ketone with C7H14O structural formula.
[2 marks]
hexane AND hex-1-ene
Accept “benzene AND hexane AND hex-1-ene”.
[1 mark]
CH3CH2CH2CH2CHBrCH3
Accept displayed formula but not molecular formula.
[1 mark]
Reagents: «concentrated» sulfuric acid AND «concentrated» nitric acid
Name of mechanism: electrophilic substitution
[2 marks]
benzene has «delocalized» \(\pi \) bonds «that are susceptible to electrophile attack» AND alkanes do not
Do not accept “benzene has single and double bonds”.
[1 mark]
curly arrow going from lone pair/negative charge on O in –OH to C
curly arrow showing Br leaving
representation of transition state showing negative charge, square brackets and partial bonds
Accept OH– with or without the lone pair.
Do not allow curly arrows originating on H in OH–.
Accept curly arrows in the transition state.
Do not penalize if HO and Br are not at 180°.
Do not award M3 if OH–C bond is represented.
Award [2 max] if wrong isomer is used.
[3 marks]
Examiners report
Phosgene, COCl2, is usually produced by the reaction between carbon monoxide and chlorine according to the equation:
(i) Deduce the equilibrium constant expression, Kc, for this reaction.
(ii) At exactly 600°C the value of the equilibrium constant is 0.200. Calculate the standard Gibbs free energy change, , for the reaction, in kJ, using sections 1 and 2 of the data booklet. State your answer to three significant figures.
(iii) The standard enthalpy change of formation of phosgene, \(\Delta H_f^\Theta \), is −220.1kJmol−1. Determine the standard enthalpy change, \(\Delta H_{}^\Theta \), for the forward reaction of the equilibrium, in kJ, using section 12 of the data booklet.
(iv) Calculate the standard entropy change, \(\Delta S_{}^\Theta \), in JK−1, for the forward reaction at 25°C, using your answers to (a) (ii) and (a) (iii). (If you did not obtain an answer to (a) (ii) and/or (a) (iii) use values of +20.0 kJ and −120.0 kJ respectively, although these are not the correct answers.)
One important industrial use of phosgene is the production of polyurethanes. Phosgene is reacted with diamine X, derived from phenylamine.
(i) Classify diamine X as a primary, secondary or tertiary amine.
(ii) Phenylamine, C6H5NH2, is produced by the reduction of nitrobenzene, C6H5NO2. Suggest how this conversion can be carried out.
(iii) Nitrobenzene can be obtained by nitrating benzene using a mixture of concentrated nitric and sulfuric acids. Formulate the equation for the equilibrium established when these two acids are mixed.
(iv) Deduce the mechanism for the nitration of benzene, using curly arrows to indicate the movement of electron pairs.
The other monomer used in the production of polyurethane is compound Z shown below.
(i) State the name, applying IUPAC rules, of compound Z and the class of compounds to which it belongs.
Name:
Class:
(ii) Deduce the number of signals you would expect to find in the 1H NMR spectrum of compound Z, giving your reasons.
The mass spectrum and infrared (IR) spectrum of compound Z are shown below:
Mass spectrum
IR spectrum
(iii) Identify the species causing the large peak at m/z=31 in the mass spectrum.
(iv) Identify the bond that produces the peak labelled Q on the IR spectrum, using section 26 of the data booklet.
Phenylamine can act as a weak base. Calculate the pH of a 0.0100 mol dm−3 solution of phenylamine at 298K using section 21 of the data booklet.
Markscheme
(i)
\( \ll {K_{\rm{C}}}{\rm{ = }} \gg \frac{{\left[ {{\rm{COC}}{{\rm{l}}_{\rm{2}}}} \right]}}{{\left[ {{\rm{CO}}} \right]\left[ {{\rm{C}}{{\rm{l}}_2}} \right]}}\)
(ii)
T«= 600 + 273» = 873K
ΔGΘ = −8.31 × 873 × ln (0.200)
OR
ΔGΘ = « + » 11676 «J»
ΔGΘ = « + » 11.7 «kJ»
Accept 11.5 to 12.0.
Award final mark only if correct sig fig.
Award [3] for correct final answer.
(iii)
ΔHΘ = −220.1 − (−110.5)
ΔHΘ = −109.6 «kJ»
Award [2] for correct final answer.
Award [1] for −330.6, or +109.6 «kJ».
(iv)
ΔGΘ= −109.6 − (298 × ΔSΘ) = +11.7 «kJ»
ΔSΘ«\(\frac{{\left( {11.7 + 109.6} \right) \times {{10}^3}}}{{298}}\)»= −407 «JK−1»
Award [2] for correct final answer.
Award [2] for −470 «JK−1» (result from given values).
Do not penalize wrong value for T if already done in (a)(ii).
Award [1 max] for −0.407 «kJ K−1».
Award [1 max] for −138.9 «J K−1».
(i)
primary
(ii)
ALTERNATIVE 1:
«heat with» tin/Sn AND hydrochloric acid/HCl
aqueous alkali/OH–(aq)
ALTERNATIVE 2:
hydrogen/H2
nickel/Ni «catalyst»
Accept specific equations having correct reactants.
Do not accept LiAlH4 or NaBH4.
Accept Pt or Pd catalyst.
Accept equations having correct reactants.
(iii)
HNO3 + 2H2SO4 NO2+ + 2HSO4− + H3O+
Accept: HNO3 + H2SO4 NO2+ +HSO4− + H2O Accept HNO3 + H2SO4
H2NO3+ + HSO4− .
Accept equivalent two step reactions in which sulfuric acid first behaves as a strong acid and protonates the nitric acid, before behaving as a dehydrating agent removing water from it.
(iv)
curly arrow going from benzene ring to N of +NO2/NO2+
carbocation with correct formula and positive charge on ring
curly arrow going from C–H bond to benzene ring of cation
formation of organic product nitrobenzene AND H+
Accept mechanism with corresponding Kekulé structures.
Do not accept a circle in M2 or M3. Accept first arrow starting either inside the circle or on the circle.
M2 may be awarded from correct diagram for M3.
M4: Accept C6H5NO2 + H2SO4 if HSO4− used in M3.
(i)
Name: ethane-1,2-diol
Class: alcohol«s»
Accept ethan-1,2-diol / 1,2-ethanediol.
Do not accept “diol” for Class.
(ii)
two AND two hydrogen environments in the molecule
OR
two AND both CH2 and OH present
(iii)
+CH2OH
Accept CH3O+.
Accept [•CH2OH]+ and [•CH3O]+.
Do not accept answers in which the charge is missing.
(iv)
oxygen-hydrogen «bond»/O–H «in hydroxyl»
\({K_{\rm{b}}} \approx \frac{{{{\left[ {{\rm{O}}{{\rm{H}}^ - }} \right]}^2}}}{{\left[ {{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{5}}}{\rm{N}}{{\rm{H}}_{\rm{2}}}} \right]}} = {10^{ - 9.13}}/7.413 \times {10^{ - 10}}\)
\(\left[ {{\rm{O}}{{\rm{H}}^ - }} \right] = \sqrt {0.0100 \times {{10}^{ - 9.13}}} = 2.72 \times {10^{ - 6}}\)
\(\left[ {{{\rm{H}}^ + }} \right] = \frac{{1 \times {{10}^{ - 14}}}}{{2.72 \times {{10}^{ - 6}}}} = 3.67 \times {10^{ - 9}}\)
OR
pOH = 5.57
pH = −log [H+] = 8.44
Accept other approaches to the calculation.
Award [4] for correct final answer.
Accept any answer from 8.4 to 8.5.